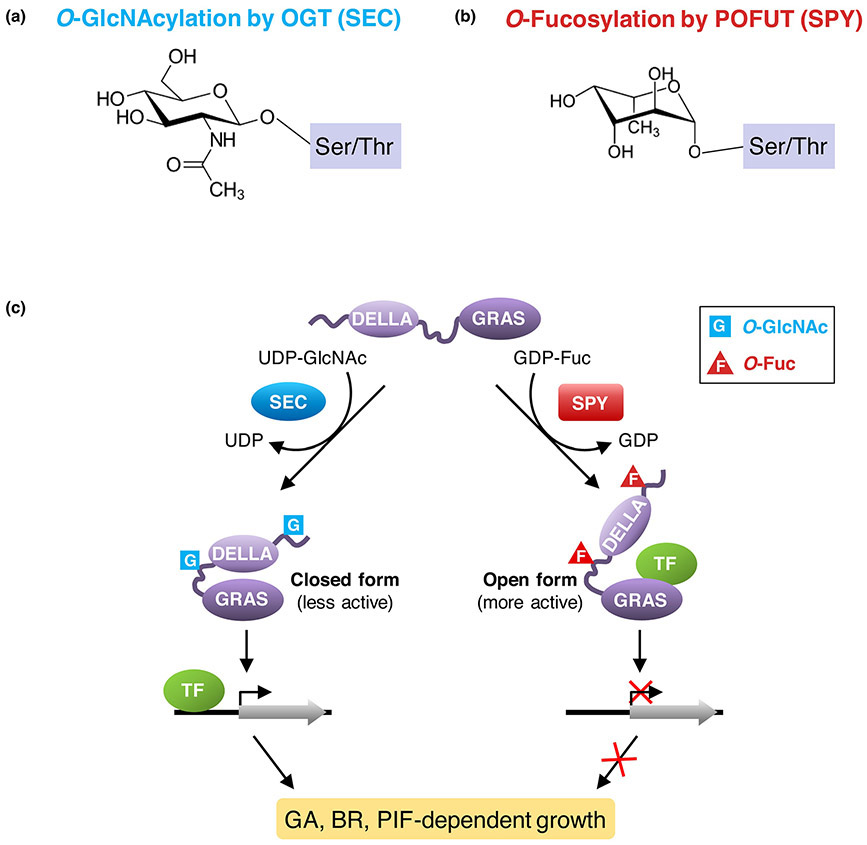
Figure 2. Model for the opposing roles of O-fucosylation and O-GlcNAcylation of DELLA in regulating plant growth.
(a) O-GlcNAcylation by OGT (SEC). (b) O-Fucosylation by POFUT (SPY). (c) The nuclear growth repressor DELLA proteins are activated by O-Fucosylation, and repressed by O-GlcNAcylation. Each DELLA protein contains an N-terminal DELLA domain and a C-terminal GRAS domain. O-Fucosylation (labeled as F) by SPY may induce the DELLA protein to an open conformation that is a more active growth repressor; this open form promotes binding of the GRAS domain to interacting transcription factors (e.g., BZR1 and PIFs), which leads to down-regulated expression of target genes of BZR1 and PIFs to restrict plant growth. In contrast, O-GlcNAcylation (labeled as G) by SEC may cause the DELLA protein to fold into a closed conformation that is less active because this form reduces its binding affinity to BZR1 and PIFs so that growth-related target genes can be activated. TF, DELLA-interacting transcription factor. The figure (c) was modified from Zentella et al. [14].