Abstract
Background:
Limited extant research on neurocognitive endophenotypes in obsessive-compulsive disorder (OCD) show inconsistent results. Limitations include small sample sizes, strict exclusion criteria, lack of objective standard normalized test scores, and significant lack of studies utilizing pediatric probands. This study aimed to address these limitations.
Methods:
A large carefully screened cohort of pediatric OCD (n=102), their unaffected siblings (n=78), and parents (n=164), completed a neuropsychological battery. To compare participants at different ages and developmental stages, standard scores were computed using test norms. Cluster-robust regression with sample size-adjusted sandwich estimates of variance, and interclass correlations were computed. False Discovery Rate procedures were employed to correct for multiplicity.
Results:
Probands, siblings and parents demonstrated deficient task performance (Z < −0.5) on the ‘number of trials to complete first category’ on the Wisconsin Card Sorting Test, and on the Stroop color naming trials. Compared to test norms, the three groups exhibited medium to large effect sizes on these outcomes. No other meaningful familial trends were found.
Conclusions:
OCD probands, their unaffected siblings, and parents exhibited deficiencies in specific subdomains of cognitive flexibility and inhibitory control, namely, initial concept formation and proactive control, which may be valid candidate neurocognitive endophenotypes of OCD. No other meaningful familial effect has been found on other functions, including other executive function indices such as perseverations and interference control. These results highlight the need to carefully examine individual outcomes from executive function tests instead of the tendency to focus largely on major outcome measures.
Keywords: endophenotype, obsessive compulsive disorder, cognitive functioning, neuropsychology, family studies
1. Introduction
Obsessive-Compulsive Disorder (OCD) is a prevalent (~2.5%; Ruscio et al., 2010) and burdensome disorder affecting children, adolescents, and adults. OCD is recognized as highly familial, with up to 10 fold increase in OCD prevalence in first degree relatives (Pauls, 2010). However, the heritability of neurocognitive deficits that may be considered as endophenotypic markers of OCD remains unclear and results have been inconsistent (Bora, 2020; Marzuki et al., 2020). Nevertheless, a recent umbrella review of biological and neurocognitive markers in OCD revealed that the only marker that showed ‘convincing’ (Class I) differential power between OCD and controls was a neurocognitive construct – visuospatial abilities (Fullana et al., 2020).
Heritability estimates of 25%−30% for OCD have been reported, with higher risk for first-degree relatives of early-onset probands (Arnold et al., 2017), but familial risk of specific neurocognitive disturbances among relatives of OCD probands remains largely inconclusive. Indeed, such research faces a fundamental limitation related to the DSM’s taxonomic approach where biological correlates are assessed in symptom-derived syndromes that are not biologically distinct. To address this problem, the National Institute of Mental Health (NIMH) developed the Research Domain Criteria (RDoC) initiative that transcends traditional DSM diagnostic categories and focuses on transdiagnostic mechanisms. However, efforts to identify clinically translatable disorders-specific neurocognitive endophenotypes utilizing the RDoC framework has not been fruitful (Davis et al., 2015; Venkatasubramanian and Keshavan, 2016). Since endophenotypes are state-independent heritable traits, they can be identified regardless of the presence of the clinical disorder (Gottesman and Gould, 2003). Thus, one venue for endophenotype research is examination of the construct in probands and their first-degree relatives. Under such framework, unaffected relatives should demonstrate a pattern of deficits that adhere closely to the co-segregated genetic risk for OCD without confounds, so that specific cognitive deficits can be reasonably considered to be genuine findings and not artefact.
There is an extensive body of literature on neurocognitive function in adult OCD that report deficient test performance across several domains (with small to medium effect sizes; Abramovitch et al., 2013; Shin et al., 2014). Specifically, OCD has been consistently associated with poorer test performance on tasks assessing planning, inhibitory control, and non-verbal memory, as well as slower processing speed (Abramovitch and Cooperman, 2015), and candidate endophenotype studies of OCD focused on such findings in probands (e.g., de Wit et al., 2012; Menzies et al., 2008). In youth with OCD, research has shown some evidence for cognitive inefficiencies (Abramovitch et al., 2015) with slower processing speed producing a negative impact on task performance (Geller et al., 2018). However, results of systematic reviews and meta-analyses concluded that effect sizes in pediatric OCD were found to be smaller than in adult OCD, with some notable differences where response inhibition and memory were not found to be meaningfully deficient in pediatric OCD (Abramovitch et al., 2015; Marzuki et al., 2020).
A small but growing body of familial neurocognitive studies in OCD shows inconsistent results. These studies have independently identified multiple candidate endophenotypes for OCD including planning, cognitive flexibility, working memory, inhibition, set-shifting, delayed verbal and non-verbal memory, problem solving, decision making under ambiguity, reversal learning, and visuospatial integration (Bey et al., 2018; Cavedini et al., 2010; de Wit et al., 2012; Delorme et al., 2007; Li et al., 2012; Menzies et al., 2007; Rajender et al., 2011; Tezcan et al., 2017; Zhang, L. et al., 2015). A recent meta-analysis (Bora, 2020) of this body of literature found that deficiencies in three executive functions (EF), namely inhibition, planning, and decision making are shared among OCD and unaffected relatives. Importantly, the authors identified significant heterogeneity between effect sizes that was unexplained by moderator analyses.
Only one study to date examined cognitive function in pediatric OCD probands (n=87) and unaffected relatives. In contrast to endophenotype research in adult OCD probands, the authors identified planning as the only candidate endophenotypic marker (Negreiros et al., 2020), echoing the differences in the extent and scope of cognitive dysfunction between pediatric and adult OCD. In addition to its inconsistent pattern of results, this body of literature has several limitations. First, most studies do not report standard scores using test norms to facilitate objective comparison of performance to the general population. Given that cognitive functions are highly dependent on neuromaturation and developmental trajectories (Levin et al., 1991), the common practice of averaging raw scores within samples comprising both children and adolescents may be less informative than utilization of standard scores. Furthermore, standard scores allow comparisons between samples of youth and adults. Second, nearly all familial neurocognitive studies in OCD employ strict exclusion criteria, most excluding some comorbid psychiatric disorders (Bora, 2020). Given that most individuals with OCD present with psychiatric comorbidities (Ruscio et al., 2010) such strict exclusion criteria pose a threat to ecological validity. Third, to our knowledge all but one investigation examining neurocognitive endophenotypes in OCD have studied unaffected relatives of adult probands. Given the higher heritability estimates among OCD youth (Arnold et al., 2017; Hanna et al., 2005), neurocognitive endophenotypic studies with pediatric OCD probands may provide more accurate familial neurocognitive information. Finally, no endophenotype study to date assessed probands and two first degree relatives, which may produce more robust results.
To address this gap in the literature, the purpose of the present investigation was to examine several neurocognitive domains in at least two first-degree relatives of pediatric OCD probands in a large well characterized sample while addressing limitations of previous research. We hypothesize that pediatric OCD probands and their relatives will present with reduced processing speed that may affect performance on timed tasks assessing higher order functions.
2. Materials and methods
2.1. Participants
Probands were 102 youth with OCD (40.5% female), that were recruited for a large family study of OCD, and 85% of probands were direct referral to the Massachusetts General Hospital Pediatric OCD Program. The remaining 15% were recruited via clinician referral, and advertisements. Inclusion criteria were primary OCD, age<18, and basic proficiency in English. Exclusion criteria included sensorimotor disability (e.g., blindness), psychosis, pervasive developmental disorder, and IQ<80. No other comorbidities were excluded due to the need to recruit an ecologically valid sample that would be analogues to the average OCD patient, where approximately 90% of adults (Ruscio et al., 2010), and 80% of youth (Geller, 2006), meet criteria for at least one other DSM disorder. Unaffected first-degree relatives (UFDR) of probands were siblings (n=78, 51.3% females), and parents (n=164, 54.9% females). Exclusion criteria for UFDR were similar to the probands exclusion criteria, with the addition of excluding lifetime OCD. Probands were predominantly Caucasian (90.5%) with other racial/ethnic groups including Asian (0.9%), Hispanic (0.9%), and other (7.7%). Probands were assessed using the epidemiological version of the K-SADS-E (Orvaschel and Puig-Antich, 1987). See Table 1. for demographic and clinical data.
Table 1.
Demographic and Clinical Characteristics of Study Participants
| Probands | Siblings | Parents | |
|---|---|---|---|
| Age | 11.5 (3.1) | 12.3 (4.9) | 44.2 (5.6) |
| Gender (female) | 40.5% | 51.3% | 54.9% |
| WRAT Reading Percentile | 69.1 (21.1) | 61.7 (19.4) | 69.4 (17.9) |
| WRAT Arithmetic Percentile | 57.0 (26.0) | 52.6 (24.3) | 61.1 (25.5) |
| CY-BOCS Total | 21.3 (5.3) | - | - |
| CY-BOCS Obsessions | 10.0 (2.9) | - | - |
| CY-BOCS Compulsions | 11.1 (2.8) | - | - |
| Medication at Time of Testing | 60.3% | 14.1% | 24.4% |
Note: Mean (SD) reported for continuous variables, and % endorsement reported for categorical variables. CY-BOCS, Children’s Yale-Brown Obsessive-Compulsive Scale; WRAT, wide range achievement test.
2.2. Neuropsychological Measures
Intellectual Ability –
Probands’ and siblings’ IQ was estimated using the vocabulary subtest of the Wechsler Intelligence Scale for Children (WISC-III; Wechsler, 1991), and parents were assessed using the Wechsler Adult Intelligence Scale (WAIS-III; Wechsler, 1997).
Processing Speed –
The WAIS/WISC-III Digit Symbol Coding, and Symbol Search subtests were used for assessment of speed of processing.
Visuospatial Abilities –
The Wechsler Block Design test, (Wechsler, 1991; Wechsler, 1997) and the RCFT Copy trial scores (Osterrieth, 1944) were used to assess visuospatial functions.
Memory –
Verbal memory was assessed using the California Verbal Learning Test (CVLT), child(Delis et al., 1994) or adult version (Delis et al., 1987). Non-verbal Memory was assessed using the delayed trial of the Rey Complex Figure Test (RCFT; Osterrieth, 1944).
Executive Functions –
Inhibitory/Interference Control was assessed using Golden’s Stroop test version (Golden, 1978). Cognitive flexibility and concept formation were assessed using the computerized version of the Wisconsin Card Sorting Test (WCST; Harris, 1990). Working memory was assessed using the Wechsler Arithmetic, and Digit Span Tests.
2.3. Procedure
Raters who were blind as to participants’ group assignment conducted a direct interview with all probands, and an independent interview with all probands’ mothers. Next, a review team reached a DSM-IV (APA, 2000) diagnosis by blindly weighting the two sources of information utilizing the Best Estimate method (Leckman et al., 1982). Subsequently, an expert child psychiatrist (DAG), administered the Children’s Yale-Brown Obsessive-Compulsive Scale (CY-BOCS; Scahill et al., 1997) and resolved any instances of discrepancy between reports. Parents were screened using the Structured Clinical Interview for DSM-IV (SCID; First et al., 2002), and modules from the K-SADS-E (Orvaschel and Puig-Antich, 1987) in order to assess conditions not included in the SCID such as separation anxiety and autism.
A team of research assistants (RAs) that received extensive training administered all neuropsychological tests under direct supervision of a PhD level staff neuropsychologist and scoring of all neuropsychological tests was performed by a second independent PhD level neuropsychologist. In addition, several measures were taken to enhance quality control. First, test administrators observed PhD-level neuropsychologist administering the test battery. Subsequently, RAs administered tests under direct supervision multiple times, until assessment competence and scoring matched the supervisors’ scores a minimum number of times. Participants signed an informed consent and were reimbursed. The study was approved by the MGH IRB.
2.4. Analytic Plan
To assess for differences in test performance between probands and their siblings and parents (i.e., differences within families), dummy-coded regression was employed. Apart from the RCFT, results that were originally obtained in the metrics of scaled scores from each test’s official norms were converted to z-scores for analysis and reporting. Probands served as the reference group, and shared family status was accounted for via cluster-robust regression, which used sample size-adjusted sandwich estimates of variance (StataCorp, 2017). To assess for variance accounted for at the family level (i.e., differences in test performance between families), intraclass correlations were calculated based on test results from each reporter (probands, siblings, parents). For the RCFT, the Waber-Holms Developmental Scoring System was used, for which normative work is available only for ages 5–14 (Bernstein and Waber, 1996). Thus, we were not able to conduct analyses of standard scores for any participants older than 14. We addressed this issue by limiting analyses to the raw scores between probands and siblings age ≤ 14 and for which proband-sibling pairs were within 1 year of age. The 1-year range was determined given that no of noticeable performance difference was found within 1-year age difference on the Waber-Holmes normative work. Hypothesis tests of regression coefficients were adjusted for multiple comparison based on the False Discovery Rate procedures (FDR;Benjamini and Hochberg, 1995). Different neuropsychological domains were considered as separate families of hypotheses for evaluation and the FDR procedure corrected for false discoveries based on the respective number of hypothesis tests for each ‘family’ including memory, visuospatial functioning, processing speed, cognitive flexibility, interference/inhibitory control and working memory. We also reported effect sizes that correspond to the various analyses conducted. According to Cohen (1988), small, medium and large effect sizes correspond to values of .2, .5, and .8 respectively for between-group differences (Cohen’s d) and values of .01, .09, and .25 respectively for measures of variance explained (R2, ICC). Cohen’s d values of .2, .5, and .8 corresponding to group differences (represented by individual regression coefficients) correspond to semipartial R2 values of .10, .24, and .37.
3. Results
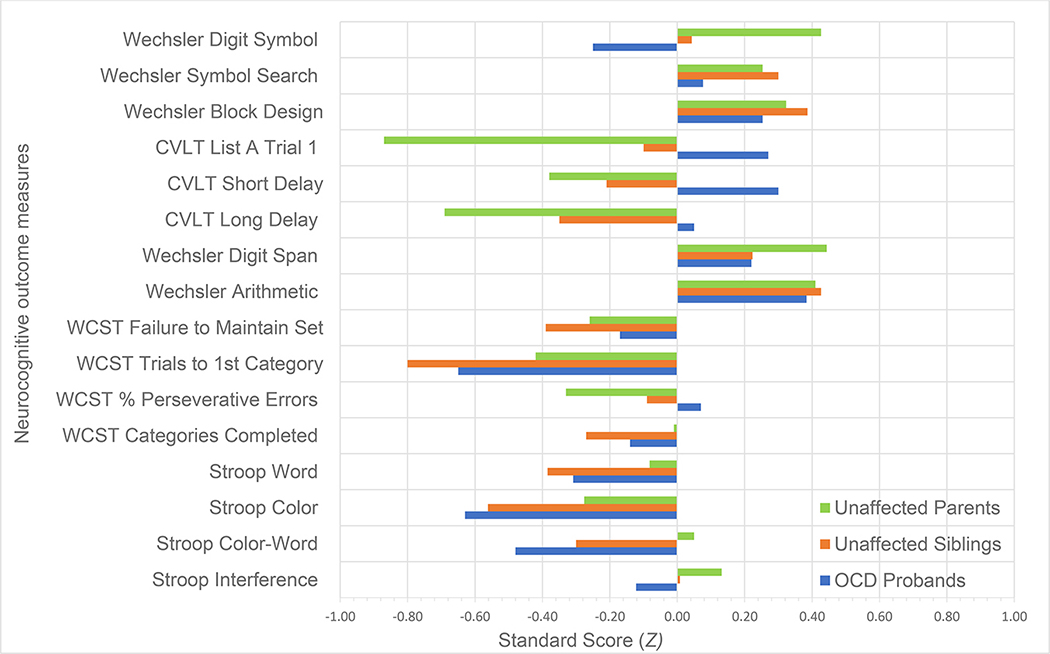
Descriptive statistics for probands, siblings, and parents can be found in Table 1. Results from comparisons between probands and their parents and siblings can be found in Table 2 and Figure 1. With regard to memory, medium to large differences were detected between probands and parents on all CVLT scales evaluated, where probands outperformed their parents on Short Delay (d=.51), Long Delay (d=.55), and List A1 (d=1.08). Small-medium to medium sized effects were also detected between probands and siblings on all CVLT scales evaluated, where probands outperformed their siblings on average on Short Delay (d=.49), Long Delay (d=.35), and List A Trial 1 (d=.34). On all CVLT scales, probands showed above-average performance (Short Delay Mean=.30; Long Delay Mean=.05, List A1 Mean=.27), while below-average performance was observed for siblings (Short Delay Mean=−.30; Long Delay Mean=−.39, List A1 Mean=−.10) and parents (Short Delay Mean=−.37; Long Delay Mean=−.68, List A1 Mean=−.87). Between-family variance was in the medium-large range for CVLT Short Delay (ICC=.19) and Long Delay (ICC=.16) but was negligible for List A1 (ICC=.01). No significant differences were observed for Rey Delay Accuracy between probands (M=45.47) and siblings (M=45.47; d<.01), with limited variance explained by between-family differences (ICC=.04).
Table 2.
Neuropsychological Test Performance: Comparison Between Pediatric Probands with OCD, Unaffected Siblings and Parents
| Probands |
Siblings |
Parents |
Parents vs Probands |
Siblings vs Probands |
Overall |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | t | p | d | t | p | d | R2 | ICC | |
| Wechsler Digit Symbol | 102 | −.25 | 1.25 | 76 | .04 | 1.15 | 163 | .45 | .78 | 5.37 | <.01* | −.71 | 1.67 | .10 | −.24 | .08 | .09 |
| Wechsler Symbol Search | 102 | .08 | 1.13 | 78 | .25 | 1.09 | 164 | .26 | .83 | 1.49 | .14 | −.19 | 1.04 | .30 | −.16 | .01 | .12 |
| Wechsler Block Design | 102 | .25 | 1.07 | 78 | .36 | .94 | 161 | .30 | .89 | .38 | .71 | −.05 | .77 | .44 | −.11 | <.01 | .10 |
| CVLT Short Delay | 98 | .30 | 1.08 | 75 | −.30 | 1.37 | 163 | −.37 | 1.41 | −4.77 | <.01* | .51 | −3.01 | <.01* | .49 | .05 | .19 |
| CVLT Long Delay | 97 | .05 | 1.15 | 78 | −.39 | 1.38 | 163 | −.68 | 1.41 | −5.20 | <.01* | .55 | −2.19 | .03* | .35 | .05 | .16 |
| CVLT List A Trial 1 | 99 | .27 | 1.13 | 78 | −.10 | 1.11 | 164 | −.87 | 1.01 | −8.27 | <.01* | 1.08 | −2.25 | .03* | .34 | .18 | .01 |
| Wechsler Digit Span | 102 | .22 | 1.01 | 78 | .22 | 1.04 | 164 | .45 | .95 | 1.68 | .09 | −.24 | .02 | .98 | <.01 | .01 | .10 |
| Wechsler Arithmetic | 102 | .38 | 1.12 | 77 | .35 | 1.05 | 162 | .43 | .85 | .47 | .64 | −.05 | −.19 | .85 | .03 | <.01 | .24 |
| WCST Failure to Maintain | 64 | .17 | 1.36 | 51 | .35 | 1.34 | 106 | .28 | 1.05 | .68 | .50 | −.10 | .75 | .45 | −.13 | <.01 | .05 |
| WCST Trials to Com. 1ST Cat. | 64 | −.65 | 1.97 | 50 | −.74 | 1.95 | 105 | −.44 | 1.31 | −.73 | .47 | .13 | .22 | .82 | −.05 | .01 | .00 |
| WCST % Perseverative Errors | 63 | −.07 | 1.29 | 50 | .15 | 1.14 | 106 | .39 | 1.23 | 2.41 | .02 | −.37 | 1.10 | .27 | −.18 | .03 | .12 |
| WCST Categories Completed | 64 | −.14 | 1.40 | 51 | −.29 | 1.36 | 106 | −.02 | 1.01 | .56 | .57 | −.10 | −.60 | .55 | .11 | .01 | .01 |
| WCST Number of Trials | 63 | −.09 | 1.30 | 51 | .18 | 1.16 | 106 | .60 | 1.06 | 3.57 | <.01* | −.59 | 1.09 | .28 | −.21 | .06 | .00 |
| Stroop Word | 99 | −.31 | .71 | 75 | −.37 | .73 | 163 | −.06 | .83 | 2.51 | .01* | −.31 | −.55 | .59 | .09 | .03 | .15 |
| Stroop Color | 99 | −.63 | .82 | 76 | −.52 | .70 | 163 | −.28 | .68 | 3.67 | <.01* | −.47 | .95 | .34 | −.14 | .04 | .11 |
| Stroop Color-Word | 99 | −.48 | .85 | 75 | −.30 | .85 | 163 | .05 | .85 | 5.06 | <.01* | −.63 | 1.40 | .16 | −.21 | .07 | .13 |
| Stroop Interference | 99 | −.12 | .64 | 74 | .04 | .69 | 163 | .14 | .67 | 3.13 | <.01* | −.40 | 1.63 | .10 | −.24 | .03 | .03 |
Note: OCD, obsessive-compulsive disorder; CVLT, California verbal learning test; WCST, Wisconsin card sorting test, Trials to Com. 1ST Cat., trials to complete first category; WISC, Wechsler intelligence scale for children; n, sample size included in the analysis; SD, standard deviation; d, Cohen’s d effect size; ICC, interclass correlation; Mean scores reflect standard scores derives from norms (Z scores)
Fig. 1.
Standard neuropsychological test scores (Z) for pediatric probands and unaffected siblings, and parents. Performance across 16 neurocognitive outcome measures is presented for OCD probands, unaffected siblings and unaffected parents. Z scores were derived from test norms. Negative values represent performance below the norm.
With regard to visuospatial functioning, no significant differences between probands and either siblings or parents were observed, with a medium-size effect observed for between-family variance (ICC=.10). No significant differences were observed for Rey Copy Accuracy between probands (M=61.74) and siblings (M=59.71), with limited variance explained by individual differences (R2=.04), though a medium-size effect was observed for between-family differences (ICC=.13).
Regarding processing speed, a significant difference was observed between parents and probands on the Digit Symbol test (d=−.71), with probands (M=−.25) underperforming their parents (M=.45). No other significant differences in processing speed were observed between probands and either parents or siblings on the Digit Symbol test or Symbol Search test. A medium-size effect for between-family variance was observed on both the Digit Symbol test (ICC=.09) and Symbol Search test (ICC=.12).
In considering cognitive flexibility, differences between probands and parents were observed on the WCST Total Number of Trials (d=−.59), with probands (M=−.09) underperforming their parents (M=.60) who completed the test using less cards. No other significant differences were observed between probands and either parents or siblings on any of the WCST scales administered. Notably, whereas no significant difference between the three groups was found on the Trials to Complete First Category, probands (M=−.65), siblings (M=−.74) and parent (M=−.44) exhibited subnormal performance of medium magnitude.
With regard to interference/inhibitory control, significant differences between probands and parents were observed on all Stroop scales administered, with probands underperforming their parents on the Word (d=−.31), Color (d=−.47), and Interference (d=−.40) Scales. Probands showed below average performance on all Stroop scales (Word Mean=−.63; Color Mean=−.48; Interference Mean=−.12), while parents also showed below average performance on Stroop Color performance (M=−.28) but average or slightly above-average scores on the Color (M=.05) and Interference (M=.14) scales. No significant differences were observed between siblings and parents on the Stroop scales. A medium size effect was observed for the Stroop Word (ICC=.15) and Color (ICC=.11) scales, but a limited amount of between-family variance was accounted for by Stroop Interference scores (ICC=.03).
For working memory, no significant differences were observed on the Arithmetic and Digit Span tests, with a large amount of between-family variance accounted for on the Arithmetic test (ICC=.24) and a medium effect size observed for between-family variance on the Digit Span test (ICC=.10).
Discussion
The aim of the present study was to identify familial patterns of cognitive deficiencies in order to identify reliable candidate cognitive endophenotypes in OCD. In terms of standardized objective performance level, to facilitate interpretations of these results we considered Z scores within +− 0.5 standard deviations from the norm as indicative of normative performance, whether performance was better or worse than the population mean. Familial deficiency was considered if the proband and at least one type of unaffected relative both scores ≤Z= −0.5. Based on these criteria intact performance was found among OCD probands and unaffected relatives on tests of working memory as well as on tests directly assessing processing speed and visuospatial functions. In the domain of verbal memory, however, unaffected siblings, and more pronouncedly parents, underperformed on immediate and delayed verbal memory trials with small and medium effect sizes, respectively. Nevertheless, OCD probands exhibited intact performance on this domain. This is in accordance with multiple studies that did not identify deficient performance on verbal memory tests, and effect sizes for verbal memory in OCD are found to be the smallest across cognitive domains (Abramovitch et al., 2013; Marzuki et al., 2020).
Examinations of the two main EF domains, namely interference control (Stroop) and cognitive flexibility (WCST), yielded an interesting pattern of results. First, the primary EF outcome measures assessing interference control (i.e., Stroop Interference) and cognitive flexibility (%Perseverative Errors, and Categories completed) were found to be largely intact, although characterized by suboptimal performance. These findings are in accord with findings from a meta-analysis on cognitive function in pediatric OCD where interference control indices indicated nearly zero effect sizes, and set shifting had a small effects (g<.3; Abramovitch et al., 2015), and with a recent meta-analysis critically examining cognitive flexibility in OCD that found no a meaningful impairment on these outcome measures (Fradkin et al., 2018). Second, the largest effects found in the present study were related to performance on secondary outcome measures from the Stroop and WCST tests. On the WCST, OCD probands and both groups of their unaffected relatives demonstrated deficient test performance on the number of ‘Trials to Complete 1st Category’, with large effects for siblings, and medium for probands. No meaningful difference between the three samples was found on standard scores. On the Stroop test, probands and unaffected relatives exhibited suboptimal performance on word, color and color-word trials, but met the criteria for deficient performance (noted above) on the Stroop color naming trial. For this outcome measure, siblings and probands exhibited deficient performance, and unaffected parents, albeit not crossing the 0.5 cutoff, exhibited suboptimal performance (z= −.28), lending additional support to the familial nature of this effect. Similar findings where differences between OCD and controls were largely attributed to the WCST and Stroop tests, were reported by others, including studies where group differences were attributed largely to the Stroop color naming trial and the WCST, in pediatric OCD (e.g., Taner et al., 2011). For example, Zhang and colleagues (2015) examined cognitive function among 40 adults with OCD, 40 unaffected siblings, and 40 controls, and found that both probands and their siblings underperformed controls exclusively on the Stroop, and WCST.
Although the WCST and Stroop generally assess cognitive flexibility and inhibitory control, to understand the constructs underlying the specific outcome measure there is a need for a high-resolution examination of the corresponding neuropsychological constructs. First, the Trials to Complete 1st category on the WCST, measures the number of cards used until 10 consecutive correct cards are placed that correctly corresponds to the first rule, and before the first rule shift on the task. This outcome is different than other outcomes because it requires the participant to understand both he nature of the task and the first governing sorting rule. Indeed very little information is provided to participants on the WCST, and administrators are instructed to read the task instruction with the following sentence: “This test is a little unusual because I am not allowed to tell you very much about how to do it…” (Heaton et al., 1993). In addition, the only feedback participants receive is whether each card was correctly on incorrectly placed. Thus, the number of cards to complete the first category on the WCST is considered a specific EF measure of the larger construct of cognitive flexibility and set shifting, termed ‘initial conceptualization’ or ‘initial concept formation’ (Heaton et al., 1993; Jodzio and Biechowska, 2010; Wiegner and Donders, 1999). The second familial findings pertain to the Stroop color naming trials. The present study utilized the Golden version of the Stroop test, in which the second trial entails sheet of paper with 100 ‘XXXX’ stimuli in red, blue and green font, and participants are required to correctly name the font color of as many stimuli as possible within a time frame of 45 second. Although somewhat less pronounced compared to the Stroop word-color condition where participants are required to ignore the name of a color and only name the font color, the Stroop Color naming trial is known to elicit the same stimulus response conflict, where there is a need to inhibit reading in order to name the color. Indeed, research shows that deficient performance on the Stroop color naming trial entail difficulties in proactive control (Littman et al., 2019). Indeed, contemporary computation theories of the Stroop task - highlighting evidence from studies manipulating proactive control - suggest that the process takes place in both incongruent trials (naming the font color in neutral words) as well as in congruent trials (naming the font color of non-words).
Overall, these results indicate a familial deficiency among probands with OCD and their unaffected relatives on initial concept formation and proactive control, both of which may be valid candidate endophenotypes. The WCST is an unusual neuropsychological test, that by design is intended to elicit a sense of ambiguity. Indeed, the standard administration instructions instruct the examiner to inform examinees that “This test is a little unusual because I am not allowed to tell you very much about how to do it…” (Heaton et al., 1993). Moreover, the WCST is known to elicit frustration, and in fact, as part of a suggested functional taxonomy of EF it has been argued that the WCST should be the primary objective measure of ‘frustration tolerance’ (Callahan, 2001). In addition, the clear element of ambiguity regarding task demands in the first stage my tap into intolerance to ambiguity in OCD. This may explain the pattern of our results where outcome measures assessing performance after the correctly identifying the initial sorting rule, were largely intact. In addition, the ambiguous nature and lack of familiarity with non-words may explain why deficiency was more pronounced than the word-color trial.
The present study has several strengths including large sample sizes, focus on pediatric probands, assessing two unaffected family members, utilizing reliable normative test data that enabled accurate calculation of standard scores, and the increased ecological validity emanating from limited exclusion criteria employed. However, the present study is not without limitations. First, this study did not assess the planning domain which has been recently proposed as a viable candidate endophenotype in pediatric OCD (Negreiros et al., 2020). However, as opposed to adults with OCD, pediatric OCD may be associated only with small effect sizes for planning (Abramovitch et al., 2015). Second, given the administration and normative data issues pertaining to the RCFT, we were not able to compare standard scores for the RCFT across groups, but only raw scores between probands and siblings of the same age range. However, although comparisons between OCD and control samples on RCFT memory trials usually produce larger effects compared to other cognitive domains in adults, in pediatric OCD aggregated effect sizes for non-verbal memory have been found to be near zero, with some studies reporting superior performance among pediatric OCD samples compared to controls (Abramovitch et al., 2015).
5. Conclusions
Results from the present comprehensive familial study of neurocognitive function in OCD suggests that deficient proactive control and initial concept formation, seen in both probands and unaffected siblings are valid candidate endophenotypes for OCD. These effect on secondary outcome measures from the WCST and the Stroop test – that are considered tests of cognitive flexibility and inhibitory control – highlight the need for a high-resolution examination of neurocognitive outcome measures, and echo the call to address the difference in construct validity between outcome measures from the same tests in OCD (Fradkin et al., 2018). In addition, rigorous familial comparative studies are needed in order to assess specificity and discriminant validity of such markers between different disorders, or different psychopathological mechanism, as per the Research Domain Criteria (RDoC) initiative.
Highlights.
A large, carefully screened cohort of pediatric OCD probands, their unaffected siblings and parents completed a neuropsychological battery.
Deficient performance was found among OCD probands and unaffected relatives on initial concept formation and proactive control.
These subdomains of cognitive flexibility and inhibitory control may be candidate endophenotypes of OCD.
These results echo the need for a high-resolution examination of secondary neurocognitive outcome measures.
Acknowledgement
The authors thank the children and families who participated in the study.
Funding
This research was supported by National Institute of Mental Health grant K08 MH01481 (to DAG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of Interest
DAG has received grant or research support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development subcontract with Duke Clinical Research Center Pediatric Trials Network, Biohaven Pharmaceuticals, Neurocrine Biosciences, Nuvelution Pharma, Peace of Mind Foundation,, Syneos Health, and Teva Pharmaceutical Industries. He has served as a consultant to the Arlington Youth Counseling Center. He has served on the editorial board of Annals of Clinical Psychiatry. He has received honoraria from the Massachusetts Psychiatry Academy and the American Academy of Child and Adolescent Psychiatry. He has held stock options/ownership in Assurex Health and Revolutionary Road. AA and ASD reported no biomedical financial interests or potential conflicts of interest.
Footnotes
The authors report no conflicts of interest pertaining to the present investigation.
Ethical Statement
The study was approved by the MGH institutional review board and all participants signed an informed consent.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramovitch A, Abramowitz JS, Mittelman A, 2013. The neuropsychology of adult obsessive-compulsive disorder: a meta-analysis. Clin. Psychol. Rev. 33(8), 1163–1171. [DOI] [PubMed] [Google Scholar]
- Abramovitch A, Abramowitz JS, Mittelman A, Stark A, Ramsey K, Geller DA, 2015. Neuropsychological test performance in pediatric obsessive–compulsive disorder – a meta-analysis. J. Child Psychol. Psychiatry 56(8), 837–847. [DOI] [PubMed] [Google Scholar]
- Abramovitch A, Cooperman A, 2015. The cognitive neuropsychology of obsessive-compulsive disorder: A critical review. Journal of Obsessive-Compulsive and Related Disorders 5, 24–36. [Google Scholar]
- APA, 2000. Diagnostic and statistical manual of mental disorders DSM-IV-TR. American Psychiatric Association, Washington, D.C. [Google Scholar]
- Arnold PD, Askland KD, Barlassina C, Bellodi L, Bienvenu OJ, Black D, Bloch M, Brentani H, Burton CL, Camarena B, Cappi C, Cath D, Cavallini M, Conti D, Cook E, Coric V, Cullen BA, Cusi D, Davis LK, (....), Zai G, 2017. Revealing the complex genetic architecture of obsessive–compulsive disorder using meta-analysis. Mol. Psychiatry 23, 1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological) 57(1), 289–300. [Google Scholar]
- Bernstein JH, Waber DP, 1996. Developmental Scoring System for the Rey–Osterrieth Complex Figure. Psychological Assessment Resources, Odessa, FL. [Google Scholar]
- Bey K, Kaufmann C, Lennertz L, Riesel A, Klawohn J, Heinzel S, Grutzmann R, Kathmann N, Wagner M, 2018. Impaired planning in patients with obsessive-compulsive disorder and unaffected first-degree relatives: Evidence for a cognitive endophenotype. J. Anxiety Disord. 57, 24–30. [DOI] [PubMed] [Google Scholar]
- Bora E, 2020. Meta-analysis of neurocognitive deficits in unaffected relatives of obsessive-compulsive disorder (OCD): comparison with healthy controls and patients with OCD. Psychol. Med, 1–10. [DOI] [PubMed] [Google Scholar]
- Callahan CD, 2001. The assessment and rehabilitation of executive function disorders, in: Brick J, Stonnington HH (Eds.), Rehabilitation of neuropsychological disorders: A practical guide for rehabilitation professionals. Psychology Press, Philadeliphia, PA, pp. 87–124. [Google Scholar]
- Cavedini P, Zorzi C, Piccinni M, Cavallini MC, Bellodi L, 2010. Executive dysfunctions in obsessive-compulsive patients and unaffected relatives: searching for a new intermediate phenotype. Biol. Psychiatry 67(12), 1178–1184. [DOI] [PubMed] [Google Scholar]
- Cohen J, 1988. Statistical Power Analysis for the Behavioral Sciences. Routledge Academic, New York. [Google Scholar]
- Davis J, Maes M, Andreazza A, McGrath JJ, Tye SJ, Berk M, 2015. Towards a classification of biomarkers of neuropsychiatric disease: from encompass to compass. Mol. Psychiatry 20(2), 152–153. [DOI] [PubMed] [Google Scholar]
- de Wit SJ, de Vries FE, van der Werf YD, Cath DC, Heslenfeld DJ, Veltman EM, van Balkom AJ, Veltman DJ, van den Heuvel OA, 2012. Presupplementary motor area hyperactivity during response inhibition: a candidate endophenotype of obsessive-compulsive disorder. Am. J. Psychiatry 169(10), 1100–1108. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kraemer JH, Kaplan E, Ober BA, 1987. California Verbal Learning Test. The Psychological Coorporation, San Antonio, TX. [Google Scholar]
- Delis DC, Kraemer JH, Kaplan E, Ober BA, 1994. California Verbal Learning Test - Children’s Version. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Delorme R, Gousse V, Roy I, Trandafir A, Mathieu F, Mouren-Simeoni MC, Betancur C, Leboyer M, 2007. Shared executive dysfunctions in unaffected relatives of patients with autism and obsessive-compulsive disorder. Eur. Psychiatry 22(1), 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). Biometrics Research, New York State Psychiatric Institute, New York. [Google Scholar]
- Fradkin I, Strauss AY, Pereg M, Huppert JD, 2018. Rigidly Applied Rules? Revisiting Inflexibility in Obsessive Compulsive Disorder Using Multilevel Meta-Analysis. Clinical Psychological Science 6(4), 481–505. [Google Scholar]
- Fullana MA, Abramovitch A, Via E, Lopez-Sola C, Goldberg X, Reina N, Fortea L, Solanes A, Buckley MJ, Ramella-Cravaro V, Carvalho AF, Tortella-Feliu M, Vieta E, Soriano-Mas C, Lazaro L, Stein DJ, Fernandez de la Cruz L, Mataix-Cols D, Radua J, 2020. Diagnostic biomarkers for obsessive-compulsive disorder: A reasonable quest or ignis fatuus? Neurosci. Biobehav. Rev 118, 504–513. [DOI] [PubMed] [Google Scholar]
- Geller DA, 2006. Obsessive-Compulsive and Spectrum Disorders in Children and Adolescents. Psychiatr. Clin. North Am. 29(2), 353–370. [DOI] [PubMed] [Google Scholar]
- Geller DA, Abramovitch A, Mittelman A, Stark A, Ramsey K, Cooperman A, Baer L, Stewart SE, 2018. Neurocognitive function in paediatric obsessive-compulsive disorder. World J. Biol. Psychiatry 19(2), 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden CJ, 1978. Stroop color and word test: A manual for clincial and experimental uses.. Stoelting Co., Chicago, IL. [Google Scholar]
- Gottesman II, Gould TD, 2003. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry 160(4), 636–645. [DOI] [PubMed] [Google Scholar]
- Hanna GL, Himle JA, Curtis GC, Gillespie BW, 2005. A family study of obsessive-compulsive disorder with pediatric probands. Am. J. Med. Genet. B Neuropsychiatr. Genet 134b(1), 13–19. [DOI] [PubMed] [Google Scholar]
- Harris ME, 1990. Wisconsin card sorting test: computer version, research edition. Psychological Assessment Resources, Odessa, FL. [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G, 1993. Wisconsin Card Sorting Test Manual: Revised and Expanded. Psychological Assessment Resources, Odessa, FL. [Google Scholar]
- Jodzio K, Biechowska D, 2010. Wisconsin Card Sorting Test as a Measure of Executive Function Impairments in Stroke Patients. Appl. Neuropsychol. 17(4), 267–277. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Sholomskas D, Thompson WD, Belanger A, Weissman MM, 1982. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch. Gen. Psychiatry 39(8), 879–883. [DOI] [PubMed] [Google Scholar]
- Levin HS, Culhane KA, Hartmann J, Evankovich K, Mattson AJ, Harward H, Ringholz G, Ewing-Cobbs L, Fletcher JM, 1991. Developmental changes in performance on tests of purported frontal lobe functioning. Dev. Neuropsychol. 7(3), 377–395. [Google Scholar]
- Li B, Sun JH, Li T, Yang YC, 2012. Neuropsychological study of patients with obsessive-compulsive disorder and their parents in China: searching for potential endophenotypes. Neurosci. Bull. 28(5), 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman R, Keha E, Kalanthroff E, 2019. Task Conflict and Task Control: A Mini-Review. Front. Psychol 10(1598). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzuki AA, Pereira de Souza A, Sahakian BJ, Robbins TW, 2020. Are candidate neurocognitive endophenotypes of OCD present in paediatric patients? A systematic review. Neurosci. Biobehav. Rev. 108, 617–645. [DOI] [PubMed] [Google Scholar]
- Menzies L, Achard S, Chamberlain SR, Fineberg N, Chen CH, del CN, Sahakian BJ, Robbins TW, Bullmore E, 2007. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain 130(Pt 12), 3223–3236. [DOI] [PubMed] [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET, 2008. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci. Biobehav. Rev. 32(3), 525–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negreiros J, Belschner L, Best JR, Lin S, Franco Yamin D, Joffres Y, Selles RR, Jaspers-Fayer F, Miller LD, Woodward TS, Honer WG, Stewart SE, 2020. Neurocognitive risk markers in pediatric obsessive-compulsive disorder. J. Child Psychol. Psychiatry 61(5), 605–613. [DOI] [PubMed] [Google Scholar]
- Orvaschel H, Puig-Antich J, 1987. Schedule for Affective Disorder and Schizophrenia for School-age Children: Epidemiologic Version: Kiddie-SADS-E (K-SADS-E). Medical College of Pensylvania; Philadelphia, PA. [Google Scholar]
- Osterrieth PA, 1944. Le test de copie d’une figure complexe; contribution à l’étude de la perception et de la mémoire. [Test of copying a complex figure; contribution to the study of perception and memory.]. Arch. Psychol. (Geneve) 30, 206–356. [Google Scholar]
- Pauls DL, 2010. The genetics of obsessive-compulsive disorder: a review. Dialogues Clin. Neurosci. 12(2), 149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajender G, Bhatia MS, Kanwal K, Malhotra S, Singh TB, Chaudhary D, 2011. Study of neurocognitive endophenotypes in drug-naive obsessive-compulsive disorder patients, their first-degree relatives and healthy controls. Acta Psychiatr. Scand. 124(2), 152–161. [DOI] [PubMed] [Google Scholar]
- Ruscio AM, Stein DJ, Chiu WT, Kessler RC, 2010. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol. Psychiatry 15(1), 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, Cicchetti D, Leckman JF, 1997. Children’s Yale-Brown Obsessive Compulsive Scale: reliability and validity. J. Am. Acad. Child Adolesc. Psychiatry 36(6), 844–852. [DOI] [PubMed] [Google Scholar]
- Shin NY, Lee TY, Kim E, Kwon JS, 2014. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychol. Med. 44(6), 1121–1130. [DOI] [PubMed] [Google Scholar]
- StataCorp, 2017. Stata Base Reference Manual, Release 15. Stata Press, College Station, Texas. [Google Scholar]
- Taner YI, Emel EB, Oner O, 2011. Impaired executive functions in paediatric obsessive-compulsive disorder patients. Acta Neuropsychiatrica 23(6), 272–281. [DOI] [PubMed] [Google Scholar]
- Tezcan D, Tumkaya S, Bora E, 2017. Reversal learning in patients with obsessive-compulsive disorder (OCD) and their unaffected relatives: Is orbitofrontal dysfunction an endophenotype of OCD? Psychiatry Res. 252, 231–233. [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian G, Keshavan MS, 2016. Biomarkers in Psychiatry - A Critique. Annals of Neurosciences 23(1), 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 1991. The Wechsler intelligence scale for children—third edition. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Wechsler D, 1997. WAIS-III administration and scoring manual. San Antonio, TX, The Psychological Corporation. [Google Scholar]
- Wiegner S, Donders J, 1999. Performance on the Wisconsin Card Sorting Test after Traumatic Brain Injury. Assessment 6(2), 179–187. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yang X, Yang Q, 2015. Neuropsychological dysfunction in adults with early-onset obsessive-compulsive disorder: the search for a cognitive endophenotype. Braz J Psychiatry 37(2), 126–132. [DOI] [PubMed] [Google Scholar]
- Zhang L, Dong Y, Ji Y, Zhu C, Yu F, Ma H, Chen X, Wang K, 2015. Dissociation of decision making under ambiguity and decision making under risk: a neurocognitive endophenotype candidate for obsessive-compulsive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 57, 60–68. [DOI] [PubMed] [Google Scholar]