Figure 1. Overview of the study design.
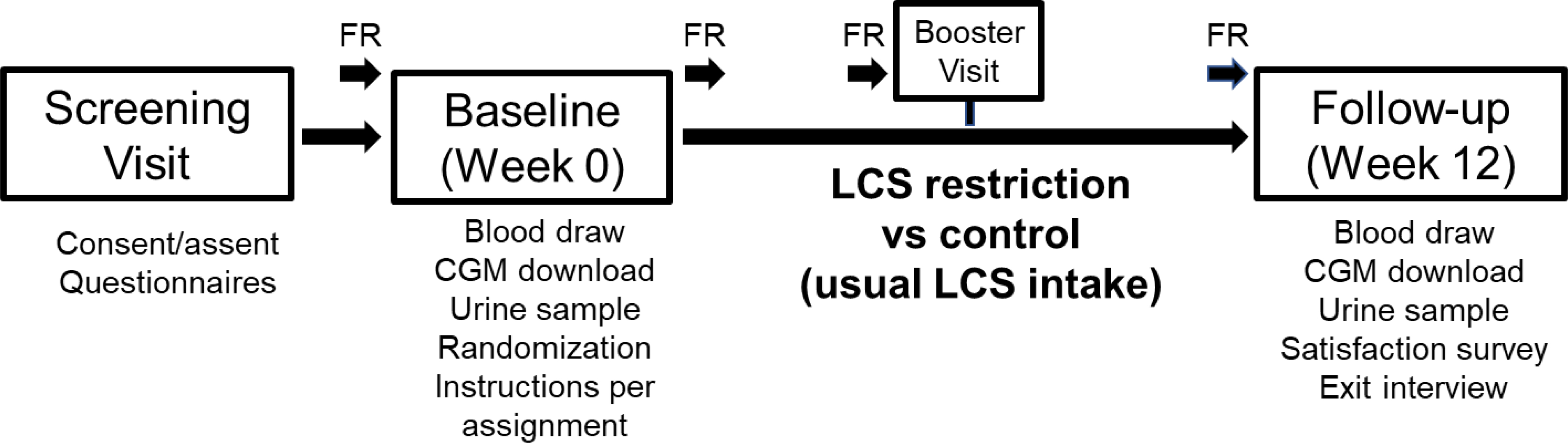
In DRINK-T1D, children with T1D are randomized to replace their usual LCSB intake with unsweetened still or seltzer water and avoid other sources of LCSs, or to continue their usual LCS consumption, for 12 weeks. Glycemic variability is monitored using continuous glucose monitoring (CGM) during a 2-week run-in period, prior to randomization, and for 2-weeks at the end of the 12-week intervention. Participants are also instructed to complete detailed, 7-day photo-assisted food records (FR) at four time points throughout the study.
