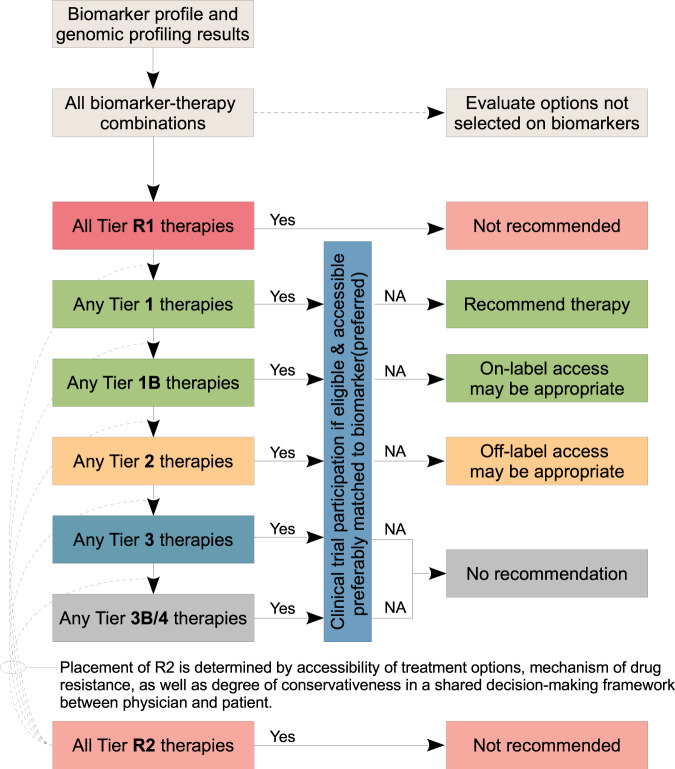
Fig. 3. Proposed cascade decision algorithm to support treatment recommendation.
In general, participation in a clinical trials should always be considered as best practice. T1 therapies are readily accessible and are thus recommended with exception of a concomitant, high-level resistance biomarker being present (i.e., Tier R1) or known treatment failure due to previous exposure, intolerance, or toxicity to another drug in the same therapeutic class. Off-label use of T2 therapies may be considered appropriate in selected circumstances. T3/4 therapies are not generally recommended outside clinical trial settings, given lack of compelling clinical data to support its use. In exceptional circumstances where treatment options are limited in rare cancer types, off-label access of lower-tier drugs may be appropriate. NA: therapy not available.