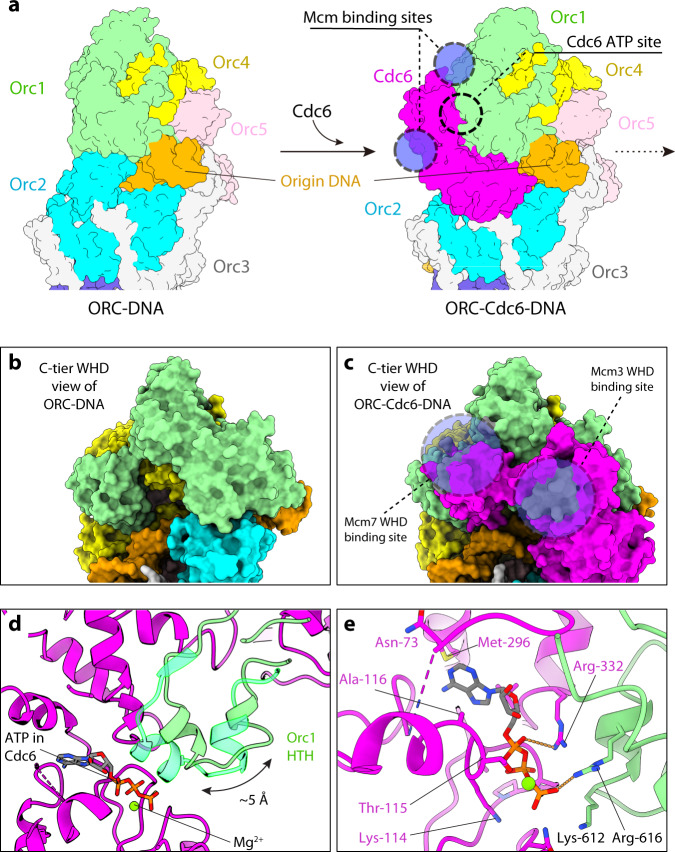
Fig. 5. Structural change in Orc1 that accommodates Cdc6 binding and activates the Cdc6 ATPase.
a Comparison of ORC (left) and ORC–Cdc6 (right) in surface view showing that the binding of the Cdc6 to ORC leads to the assembly of three composite Mcm2-7 binding sites, which are absent in ORC itself. b, c Enlarged view of panel a showing the absence (b) and the presence (c) of the binding sites (dashed black circles) for the WHDs of Mcm3 and Mcm7. d A short α-helix of Orc1 AAA+ domain shifts by 5 Å upon Cdc6 binding to form the ATP-binding pocket of Cdc6. e The Cdc6 ATP binding site is formed by side chains from both Cdc6 and Orc1. Orc1 Arg-616 serves as the arginine finger for the ATP in Cdc6. ATPγS and surrounding residues are shown as sticks.