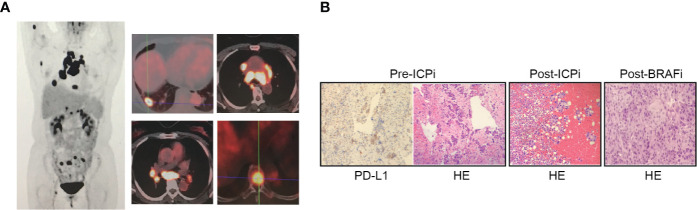
Figure 1.
PET/CT imaging and histological finding. (A) PET/CT imaging on 2018/03/19 revealed an enlarged nodule measuring 2.0 × 1.6 cm on right lower lobe (RLL) with SUVmax 8.4, bilateral supraclavicular lymph nodes (0.5 cm and 0.6 cm on the right and left sides respectively) with SUVmax 2.8, enlarged mediastinal lymphadenectases including station 2R, 4R, 4L, 3A, 3P, 5, 6, 7, and right hilar lymphadenectasis with SUVmax 26.4 of the largest diameter of 4.2 cm, multiple bony metastases including right humerus, right scapula, T5 and T8 vertebral body, multiple sacrum, right acetabulum, right sciatic bone with a SUVmax 7.8. (B) Histological examination on the metastatic subcarinal lymphadenectasis tissue of pre-ICPi (first-biopsy), pericardial effusion of post-ICPi/pre-BRAFi (second-biopsy), and left supraclavicular lymphadenectasis of post-BRAFi (third biopsy) (hematoxylin and eosin stain, magnification ×100) and programmed death ligand-1 (PD-L1) expression in pre-ICPi (immunohistochemical stain, magnification ×100; E1L3N, Cell Signaling Technology, Danvers, MA); poorly differentiated lung adenocarcinoma positive for CK, TTF1, and NapsinA, negative for CD56 and P40 by immunohistochemistry stain in pre-ICPi (03/28/2018), post-ICPi/pre-BRAFi (12/20/2019), and post-BRAFi (06/04/2020). ICPi, immune check-point inhibitor; BRAFi, BRAF inhibitor.