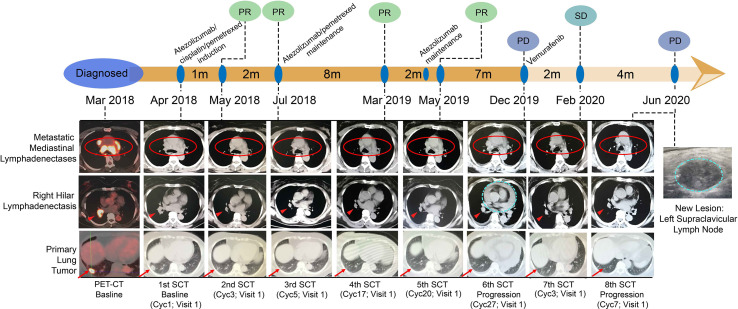
Figure 2.
Course of the disease, treatment history, and response evaluation. 1st surveillance CT (SCT): baseline, atezolizumab/cisplatin/pemetrexed induction (Cyc1, 2018/04/11); 2nd SCT: post-induction two cycles (Cyc3, 2018/05/28), PR; 3rd SCT: post-induction four cycles, atezolizumab/pemetrexed maintenance (Cyc5, 2018/07/12), PR; 4th SCT: post-atezolizumab/pemetrexed maintenance 12 cycles (Cyc17, 2019/03/25), PR; 5th SCT: post-atezolizumab maintenance one cycle (Cyc20, 2019/05/27), PR. NOTE: Atezolizumab maintenance on C19V1. Tumor assessment every 9 weeks (three cycles) after the completion of the week 48 tumor assessment per protocol (Cyc20, 2019/05/27); 6th SCT: post-atezolizumab maintenance eight cycles (Cyc27, 2019/12/17), PD; 7th SCT: post-Vemurafenib two cycles (Cyc3, 2020/02/03), SD; 8th SCT: post-Vemurafenib six cycles (Cyc7, 2020/06/04), PD. Red circles: metastatic mediastinal lymphadenectases; Red short arrows: right hilar lymphadenectasis; Red long arrows: primary lung tumor; Blue dotted cycle: new lesion; lesion 1: pericardial effusion (progression on ICPi); lesion 2: left supraclavicular lymph node (progression on BRAFi). PR, partial response; SD, stable disease; PD, progressive disease; ICPi, immune check-point inhibitor; BRAFi, BRAF inhibitor.