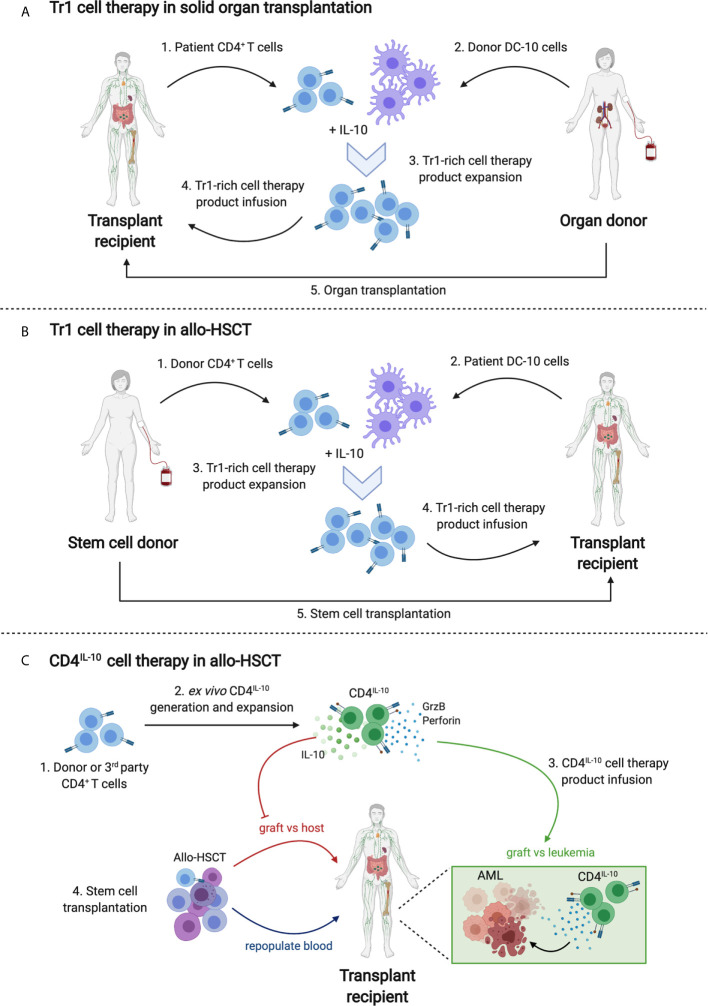
Figure 1.
Tr1 cell therapy strategies in transplantation. (A) Tr1 cell therapy in solid organ transplantation. Patient CD4+ T cells (1) and donor tolerogenic DC-10 cells (2) will be isolated prior to organ transplantation. A Tr1-rich cell therapy product will be expanded by culturing CD4+ T cells and DC-10 in the presence of exogenous IL-10 (3). The alloantigen-specific Tr1-rich cell therapy product will be phenotyped and tested ex vivo for suppressive capacity and will be infused into the transplant recipient (4). Concurrently or a day after infusion, organ transplantation will take place (5). (B) Tr1 cell therapy in allo-HSCT. Donor CD4+ T cells (1) and patient-derived tolerogenic DC-10 cells (2) will be isolated prior to HSCT. Steps (3) and (4) will be followed as in part (A). A day after infusion, HSCT will take place (5). (C) CD4IL-10 cell therapy in allo-HSCT. In this example, the transplant recipient is an acute myeloid leukemia patient. Stem cell donor or 3rd party healthy donor CD4+ T cells will be isolated (1) and transduced with hIL10 lentivirus to generate CD4IL-10 cells (2). After ex vivo expansion and quality control, CD4IL-10 cells will be infused to the transplant recipient (3) concurrently with allo-HSCT (4). While donor stem cells repopulate blood, donor T cells promote graft versus host disease (GvHD). CD4IL-10 cells prevent GvHD by secretion of IL-10 and promote graft versus leukemia (GvL) response via granzyme B/perforin mediated cytotoxicity against residual AML cells.