FIGURE 6.
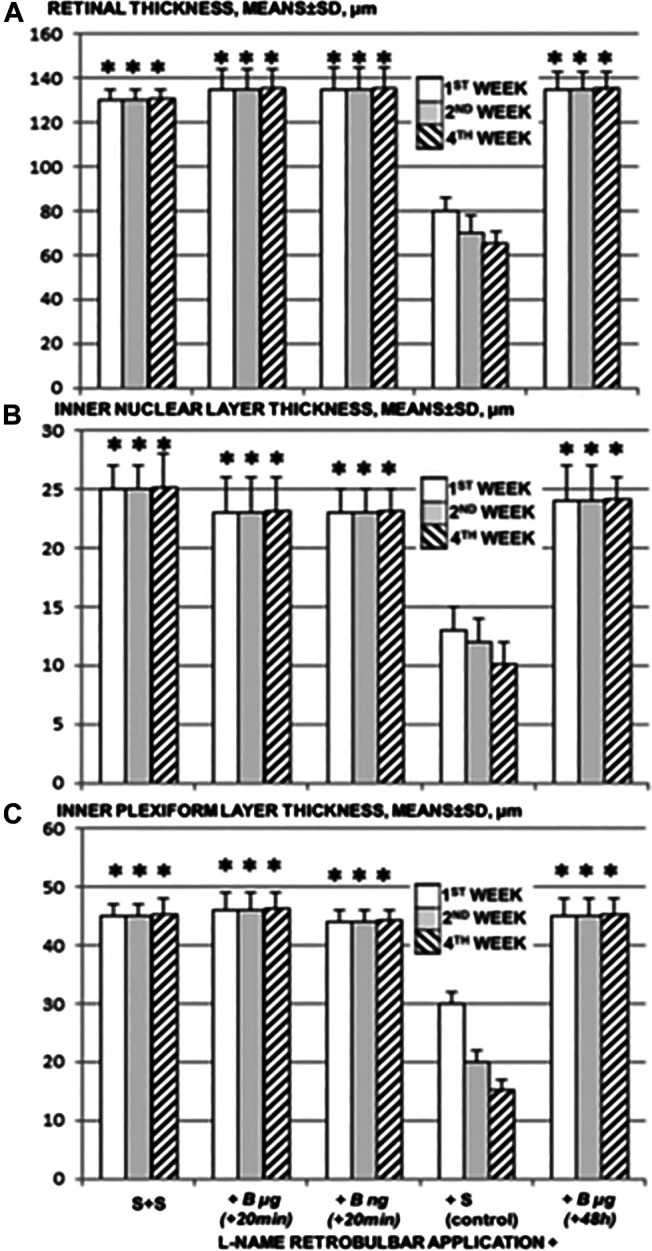
Retrobulbar L-NAME application–induced retinal damage. Retinal thickness (A), inner nuclear layer thickness (B), and inner plexiform layer thickness (C) presentation after retrobulbar agent’s application. Left. Saline retrobulbar application(s) completely preserved retinal thickness (S + S). Right. L-NAME retrobulbar application (total dose 5 mg/kg, which was divided by retrobulbar application in each eye, 0.5 mg/0.1 ml saline/eye) (L-NAME retrobulbar application +). Rats underwent L-NAME that received BPC 157 therapy {total dose 10 μg/kg or 10 ng/kg as retrobulbar application in each eye, 1 μg/0.1 ml saline/eye or 1 ng/0.1 ml saline/eye) at 20 min [B μg (+20 min), B ng (+20 min)], or 48 h [B μg (+48 h)]}. Preserved retinal thickness, inner nuclear layer thickness, and inner plexiform layer thickness. Rats underwent L-NAME that received saline as therapy [saline only as a retrobulbar application in each eye (0.1 ml/eye)] [since no different, the rats underwent L-NAME that received saline retrobulbar application and were presented together (+S (control)]. Less retinal thickness. *p<0.05, at least vs. control. 10 rats per experimental group and period for all experiments.
