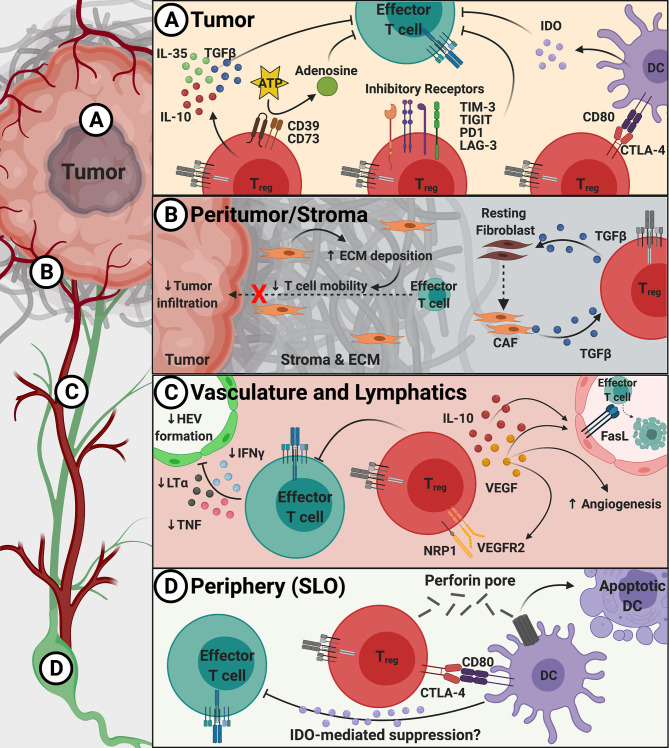
Figure 1.
Overview of suppressive mechanisms used by Tregs to create barriers to immune infiltration into tumors. Panel (A) Within the TME, Tregs utilize inhibitory receptors (TIM-3, TIGIT, PD1, and LAG-3), inhibitory cytokines (TGFβ, IL-10, and IL-35), DC modulation (via CTLA-4 and LAG-3), and metabolic disruption (via CD39/CD73) to suppress the anti-tumor T cell response. (B) Treg-derived TGFβ induces cancer-associated fibroblast (CAF) development that increases extracellular matrix (ECM) production and deposition within the peritumoral space (stroma) to inhibit effector T cell migration. (C) Tregs block entry of effector T cells through preventing proper cytokine signals that promote high endothelial venule (HEV) formation as well as production of inhibitory IL-10 and VEGF to promote dysregulated angiogenesis. (D) In the periphery and secondary lymphoid organs (SLO), Tregs can modulate DC maturity and induce apoptosis to prevent proper effector T cell activation.