Abstract
Aim
The effect of reopening schools on children's contribution to SARS‐CoV‐2 transmission, especially within households, remains controversial. This study describes the clinical presentation of a large ambulatory COVID‐19 paediatric cohort and evaluates the role of children in household transmission prior to and following school reopening.
Methods
A retrospective database cohort study was conducted in a large Health Maintenance Organization in Israel. Data of all paediatric, laboratory‐confirmed Coronavirus cases between 28/2/2020 and 20/6/2020 were extracted. All cases were analysed for household contacts and primary cases within each family cluster.
Results
A total of 1,032 cases under 18 years old (median age 12 years) were included. Of these cases, 432 (41.9%) were asymptomatic; 122 (11.8%) cases acquired the infection at school, and 45 of them were part of two school clusters; 846 children had at least one positive household contact, in 498 family clusters, and among them, 293 primary cases were identified. Only 27 (9.2%) primary cases were under 18 years of age and six (2%) were below 10. The proportion of primary cases did not change after the re‐opening of educational facilities.
Conclusion
Children, particularly under 10 years of age, are less likely to be the vector for SARS‐CoV‐2 infection within household settings. Opening educational facilities did not change transmission dynamics.
Keywords: children, COVID‐19, household, school, transmission, ultra‐orthodox
Abbreviations
- COVID‐19
Coronavirus Disease 2019
- SARS‐CoV‐2
Severe acute respiratory syndrome coronavirus 2
- MHS
Maccabi Healthcare Services
- MoH
Ministry of Health
Key notes.
This study describes the clinical presentation of a large ambulatory COVID‐19 paediatric cohort in Israel and evaluates the role of children in household transmission prior to and following school reopening during February–June 2020.
All cases were analysed for household contacts and primary cases within each family cluster.
Children were less likely to be the source for SARS‐CoV‐2 infection within households, while opening educational facilities did not change transmission dynamics.
1. INTRODUCTION
Children infected with SARS‐CoV‐2 tend to have milder disease and a very good prognosis. 1 , 2 , 3 Most reports regarding children are based on hospital settings 4 rather than the ambulatory setting. At the beginning of the pandemic, many children with COVID‐19 were infected as part of a family cluster with the disease. 5 , 6 As the disease spread globally and data accumulated, the issue regarding the extent of the children's contribution to COVID‐19 transmission remained controversial. 7 , 8
Israel, like many other countries, closed all its educational facilities on March 13th as a mitigation measure. Following on from the closure, Israel is one of the few countries that had re‐opened its educational facilities quickly, beginning on May 3rd until May 17th, by which time all facilities were opened nationally, simultaneously together with the opening of most of the economy. The Israeli ministry of health required all educational facilities to comply with hygiene, social distancing regulations and learning in small group capsules (15 students), in separate classrooms. In addition, children were required to hand in a daily health statement signed by a parent. Facemasks were required during class for students from fourth grade and above, and out of class for all the students from the first grade. High schools (7th–12th grade) were closed due to summer break from June 20th, and elementary school from July 1st. A major concern was that children will contract COVID‐19 infection within the school settings and transmit it to family members, thus, exposing adults who are at higher risk of developing severe disease. A second wave of COVID‐19 occurred around the middle of June, and raised the question as to the possible effect of school openings on infection rates of children and their families.
The aims of this study were to describe the clinical presentation of a large ambulatory COVID‐19 paediatric cohort, to estimate the risk of exposure to COVID‐19 in the school setting and to determine the role of children in the household transmission of COVID‐19 before and after school reopening.
2. MATERIALS AND METHODS
We conducted a retrospective cohort study using data from a single healthcare provider.
Data sources were the extensive central databases of Maccabi Healthcare Services (MHS). MHS is the second‐largest not‐for‐profit, sick fund in Israel, which serves over 2.6 million (27%) Israeli citizens. Membership in sick funds is compulsory in Israel, and by the National Health Insurance Law of 1994, all citizens must freely choose one of four national sick funds that are prohibited by law from denying membership to any Israeli resident. Dataset included demographic and clinic data as well as laboratory data from a single central laboratory. The study population consisted of all MHS members between the ages of 0–18, laboratory‐confirmed SARS‐CoV‐2 infected children, diagnosed between 2/28/2020–6/20/2020.
Real‐time reverse transcription‐polymerase chain reaction testing using both nasopharyngeal and throat swabs confirmed SARS‐COV‐2 infection. Criteria for test performing were according to Israel's Ministry of Health (MoH)'s guidelines, 9 based on a combination of symptoms suggestive of COVID‐19 as judged by physicians, a history of close contact with confirmed cases, or those who travelled abroad in the past 14 days. Asymptomatic cases were tested as part of positive case contact tracing in school and household settings.
Once a positive SARS‐CoV‐2 test was identified, it was followed up by a central remote COVID‐19 centre until recovery and a formal discharge, according to the Israeli MoH's policy. A structured admission process was developed, which included required questions about symptoms, date of symptoms onset and duration of symptoms; the presumed source of infection—person, place and time of exposure; household size (number of people who usually live in the house) and household contacts.
Household contacts of SARS‐CoV‐2 confirmed cases were put in quarantine and tested during the observation period, usually by the fifth day of exposure, according to the Israeli Ministry of Health's policy. 9 Those who tested negative, were monitored for symptoms during quarantine, and were retested if they exhibited symptoms.
To estimate secondary infection rates by the age of infector and age of infectees, we defined a household contact as a person who lived in the household of a COVID‐19 confirmed patient. Family members were followed up for 2 weeks after the diagnosis. After identification of all households with more than one COVID‐19 paediatric case, we determined a primary case within it. A primary case was considered the first person with a confirmed diagnosis of SARS‐CoV‐2 infection in the household and as the presumed source of secondary infection within the household. We defined the date of ‘infection onset’ for every household case as the date of developing the first symptom. For asymptomatic cases, we considered the date of test performance. The first SARS‐CoV‐2 confirmed case in the household was labelled as the primary case only if the onset of infection was at least 3 days prior to other household contacts; otherwise, the source of infection was presumed to be external. We evaluated the rate and proportion of index primary cases before and after the opening of the educational facilities.
Data collected included demographics, socio‐economic, comorbidities (based on chronic diagnoses and relevant chronic medication purchase) and outcomes. Clinical symptoms and the source of infection were based on patients' and/or parents' self‐reports. Children who did not develop any symptoms during the observation period were labelled as asymptomatic. Data were obtained from the electronic medical record and hospital discharge notes and were reviewed by one of the authors (SSBD).
Data were analysed using descriptive statistics and Student's t‐test. A chi‐square test of independence was performed to examine the relationship between age groups, and the independent variables. The local MHS ethics committee and institutional review board approved the study (approval number: 0023‐20MHS).
3. RESULTS
From February 28th until June 20th 762,707 children under the age of 18 were insured in MHS, 31.7% of all MHS' members. 5,513 MHS patients (age range 0–97 years) were identified as confirmed COVID‐19 cases. Paediatric patients constituted 1032 cases aged 0–18 years (18.7% of all cases) and were all included in this study. Their median age was 12 {IQR 7, 15} and 44 (4.2%) were infants under one year of age. 496 (48.1%) were females (Table 1) and 129 (12.5%) had a comorbidity: Obesity (body mass index 30 or above, 19,2.4%), asthma (18,1.7%), immunosuppression (6,0.6%), diabetes mellitus (2,0.2%), congenital heart disease (15,1.5%), coeliac (6,0.6%), psychiatric disorder (9,0.9%) and attention deficiency and hyperactive disorder (70,6.8%). Most children lived in the central district of Israel (Tel Aviv area, 575, 55.7%) and belonged to an Ultra‐orthodox Jewish community (55.5%).
TABLE 1.
Demographics, clinical and Epidemiological Characteristics of Paediatric Patients with COVID‐19
| Age |
0–9years N = 365 |
10–18years N = 667 |
0–18years N = 1032 |
|---|---|---|---|
| Sex‐Female n (%) | 191 (52.3) | 306 (45.8) | 496 (48.1) |
| Socio‐economic status n (%) n = 1029 a | |||
| 1–3 | 125 (34.2) | 230 (34.5) | 355 34.4) |
| 4–6 | 169 (46.3) | 333 (50) | 502 (48.6) |
| 7–10 | 68 (18.6) | 104 (15.5) | 172 (16.6) |
| Households members per family n (%)n = 830 b | |||
| 5 or less | 140 (44.9) | 129 (24.9) | 269 (32.4) |
| More than 5 | 172 (55.1) | 389 (75.1) | 561(67.6) |
| Residential community n (%) c | |||
| Ultraorthodox Jews | 175 (47.9) | 397 (59.6) | 572 (55.5) |
| Non Ultraorthodox Jews | 156 (42.7) | 218 (32.6) | 374 (36.2) |
| Arabs | 34 (9.3) | 52 (7.8) | 86 (8.3) |
Comparison between patients according to age groups using a chi‐square test. Chi‐square test was performed when n in both groups was 5 or more.
p < 0.001.
p < 0.05.
Residential area socio‐economic status on a scale from 1 (lowest) to 10.
Four hundred and thirty‐two(41.9%) cases were asymptomatic throughout the whole observation period. Among the symptomatic patients, fever of 38,0°C or above and cough were the most frequent symptoms (207%, 34.5% and 185, 30.8% respectively), followed by headache (102, 9.9%), taste and/ or smell disturbance (94, 9.1%), sore throat (83, 8%), weakness (83, 8%), rhinorrhoea (65, 6.3%) myalgia (59, 5.7%) diarrhoea (47, 4.5%) and subjective shortness of breath (17, 1.6%). Patients reported a median duration of symptoms of three (IQR {1, 6}, n = 572) days prior to testing for SARS‐COV‐2.
All cases had been monitored by the MHS' remote treatment centre during their isolation. Only 24 patients (2.3%) with a median age of 13 (IQR 8, 16), were admitted to the hospital. Reasons for admission included the need for quarantine at the beginning of the pandemic (6), psychosocial (4), fever (3), vomiting and dehydration (3), chest pain (3) dyspnoea (2), restlessness (2) and new‐onset diabetes (1). They were all stable, none of them needed ICU admission or respiratory support and none were treated with COVID‐19‐targeted therapy or steroids. The median length of stay in the hospital was two (IQR 1, 7) days. All 1032 paediatric COVID‐19‐positive cases were considered recovered according to the Israeli MoH recovery criteria. 9 During the 2 months follow‐up, none of the children required re‐admission.
3.1. Epidemiology
The most common presumed source of SARS‐CoV‐2 infection was contact with a confirmed COVID‐19 case (76.9%), followed by school exposure (11.8%), and an unknown source (8.4%). Among the 794 children who acquired the infection by a confirmed case, 602 (75%) were infected by a parent, 72 (9%) by a sibling, of which 50 siblings were over the age of 17 years. Among the 122 patients who acquired the infection within the school setting: two were at day care (3–35 months old), 12 in preschool (3–6 years old), 47 were at elementary school (1st–6th grade) and 61 were in high school (7th–12th grade). More than half of them (67, 54.9%) were asymptomatic and screened as part of contact tracing. Although most cases were sporadic from different areas and sectors in Israel, 45 cases were part of two clusters: A cluster of 29 students from an elementary school belonging to the Arab community in Jaffa in 3rd to 6th grades and a cluster of 16 students from a high school in Jerusalem in 7th–10th grades 10 (Figure 1).
FIGURE 1.
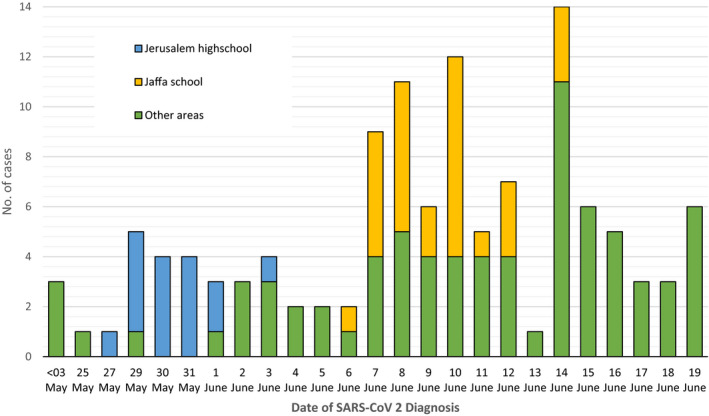
Epidemic curve of children Infected with SARS‐CoV‐2 at school settings. School settings—Day care (3–35 months old, n = 2), Preschool (3–6 years old, n = 12), Elementary school (1st–6th grade, n = 47), High school (7th–12th grade, n = 61). May 3rd—The date of school settings gradual reopening
3.2. Potential for disease transmission in household settings
Ninety‐five percentage of 1032 COVID‐19 paediatric cases' family members were screened for SARS‐CoV‐2 as part of contact tracing in a household setting. In this cohort, 846 children had at least one family member infected with SARS‐CoV‐2, in 498 households. Among them, 293 primary cases were identified, 27 were children under the age of 18 years, and 266 were adults. In all, 452 children were identified as secondary cases within the household (Figure 2). The average additional SARS‐CoV‐2 cases in family members following the introduction of the infection by an adult was 2.86, compared to 1.77 by paediatric primary case. Only 6 (2%) primary cases were under the age of 10 years. The median number of SARS‐CoV‐2 confirmed cases per household with a paediatric case was 4 (IQR 2, 5). 192 (18.6%) children did not have an additional positive SARS‐CoV‐2 case within their household 48 of them were under 10 years of age. With the educational facilities opening since May 3rd, the rate of paediatric primary cases did not increase, and their proportion did not change statistically significantly (8.7% before and 11.1% after, p = 0.557). Of the 122 children who acquired the infection within the school setting, six (5%) resulted in secondary cases in the households of the school exposed, two from elementary school and four from high school.
FIGURE 2.
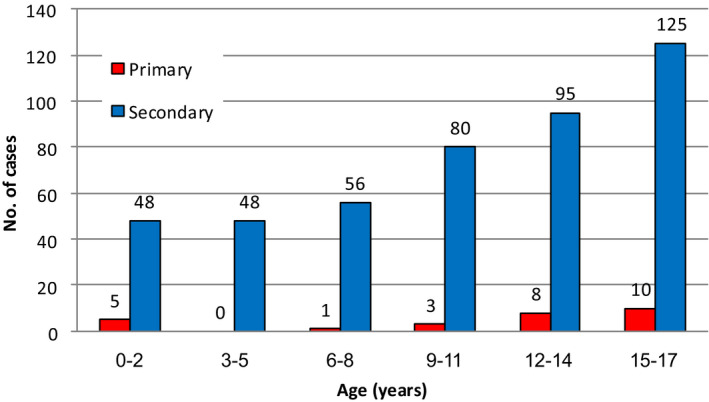
Number of primary and secondary infected cases identified within a household according to age groups. Primary case—The first person with a confirmed diagnosis of SARS‐CoV‐2 infection in the household. A primary case is the presumed source of secondary infection within the household. A minimum of 3 days interval between the primary and successive cases was defined
4. DISCUSSION
We present findings from a large ambulatory paediatric COVID‐19 patient cohort, throughout the first wave of the COVID‐19 pandemic in Israel and the beginning of the second. All children in our cohort recovered with no complications during the isolation period and 2 months thereafter. The presumed source of infection for the majority of our paediatric cases was close contact with another COVID‐19 case, usually a parent.
Understanding the proportion of children infected with SARS‐CoV‐2 is important for evaluating their part in the spread of the epidemic in the community. The proportion of paediatric cases in our study is higher than reported in other studies. 11 , 12 One possible reason for this high proportion is the overall higher proportion of children in the Israeli population than in other developed countries. 13 Other main reasons are the high rate of infection in the ultra‐Orthodox community, which is characterised by younger and larger families who share dense living conditions, and also the higher rate of asymptomatic paediatric cases—41.9% in this cohort. 3 , 14 , 15 As contact tracing in household and school settings was performed in Israel, 9 , 10 a high percentage of asymptomatic or presymptomatic‐infected children was found. This finding is in line with the study by Poline J et al. which reported up to 45% of children who were screened when admitted to hospital were asymptomatic. 16 The high proportion of asymptomatic cases is particularly important because it may reflect more realistically the paediatric SARS‐CoV‐2 confirmed cases’ clinical characteristics.
Most of the children in this cohort live in a large household and belong to the ultra‐orthodox Jews community, which has a much higher SARS‐CoV‐2 infection rate than their proportion in the general Israeli population. 17 Previous data have revealed that children from ethnic minorities appear to be disproportionately affected. 18 Reasons for the high incidence of COVID‐19 in this community may be due to dense living conditions in self‐segregated populations and the difficulty to keep proper quarantine conditions; a large number of contacts needing to be traced for every case; limited communication with authorities on Shabbat and Jewish holidays which may delay the SARS‐CoV‐2 test performance and thus, the delay required isolation.
Households are major platforms for SARS‐CoV‐2 transmission. 19 An Israeli report regarding the same time period and similar population of Ultra‐Orthodox Jews found a lower rate of child infectivity with SARS‐CoV‐2 compared with adults residing in the same households. 20 We explored the transmission potential of children within households by tracing primary cases in the paediatric cases' households 21 . The minority of paediatric primary cases in our large ambulatory cohort supports the concept that children are less often the drive to family transmissions of SARS‐ CoV‐2. The low ratio of paediatric primary cases, particularly of children under 10 years of age, is in correlation with a study from Posfay‐Barbe KM et al. that demonstrated that children are usually not the initial case in most households. 22 Another supportive finding for the conclusion that younger children are less infectious than adolescents is the fact that 48 children, under ten years of age, did not have a household contact infected, despite the limited ability to maintain isolation in this age group. Furthermore, our results show that within the household, an adult primary case will infect more members of the family than a child primary case, at a ratio of 1:1.6.
As schools reopened in May, a major concern was the role of school settings in COVID‐19 transmission. In our study, 11.8% of the paediatric population acquired the infection at a school setting, of which, two‐thirds were from different schools and areas and a third were part of two clusters. 10 Our findings demonstrate that school transmission is possible but, usually in a sporadic manner, rather than in a systemic way and outbreaks are usually the exception. 23 This is in line with previous reports describing the super‐spreading characteristics of SARS‐COV‐2, whereby the minority of infected individuals spreads the virus onwards, thus representing inhomogeneous spreading. 24 The most susceptible group for acquired transmission at school is the adolescent group. Hence, this finding might reduce reluctance to opening schools for younger children, and instead consider differential solutions for different age groups. As more than 50% of cases who acquired the infection at school were asymptomatic, reducing the burden of unrecognised asymptomatic viral spreaders by contact tracing 25 and putting in quarantine school contacts is crucial in order to stop potential COVID‐19 dissemination.
The impact of reopening educational facilities on household transmission and the community is still controversial. Somekh et al. found a gradually increased incidence of SARS‐CoV‐2 infections following school reopening in Israel between March and July 2020, especially in adults compared to children. 26 Another study from Utah demonstrated transmission from 12 young children who acquired the infection in child care settings to some of their household members, 27 whereas a study from Paris found that day care is not major foci for viral transmission. 28 In our research, which included 2 weeks follow‐up for every case, the secondary household transmission of SARS‐CoV‐2 among children who acquired the infection within the school setting occurred in only six (5%) cases. When evaluating the transmission dynamics within families before and after the re‐opening of educational facilities, the proportion of paediatric primary cases did not change significantly. Hence, we suggest that re‐opening educational facilities, did not play a significant part in the evolving pandemic in household settings. Proper Infection control measures including personal hygiene, wearing a mask, social distancing and also proper ventilation 29 , 30 as well as contact tracing and quarantine of all school and household contacts would probably minimise the possibility of viral spread within educational facilities.
Our study has several limitations. First, we present observational data from a large community database, which was partly based on telephone reports from the patients. Therefore, the information relies on patient perception for the presumed source of infection, and cannot be completely validated.
However, skilled medical personnel who were responsible for the admission process to a remote monitoring centre obtained the telephone reports, and the data were recorded in the medical files as part of the admission records.
Second, as children tend to be more asymptomatic than adults, or present with mild disease that might be overlooked, the actual rate of infection and viral carriage is potentially underestimated. This can lead to potential bias in identifying primary cases from schools or within a family.
Third, the duration of follow‐up during the study period was relatively short since schools were re‐opened during a period of low infection rate, therefore more household transmission or school cases may have been limited. A longer period of follow‐up since school opening may increase primary cases from the school settings.
Finally, the study refers to the period 28 Feb–20 Jun 2020, reflecting the virus characteristics at that time period. The B.1.1.7 lineage, which emerged in Israel in late 2020, 31 being more transmissible also among children 32 is not studied in this research. Therefore, conclusions are not generalisable to other virus variants with other characteristics.
The most prominent strength of our nationwide research is its large sample size. Other strengths are extensive household contact tracings, a 2 weeks follow‐up for cases' household members in order to avoid lag time bias and the absence of patients lost to follow‐up.
5. CONCLUSIONS
Our data suggest that children usually acquired SARS‐CoV‐2 infection at home but are less likely to be the vector for the infection within the household. Opening educational facilities did not change the transmission dynamics in the households. Transmission within household settings should be considered when designing strategies to mitigate the COVID‐19 pandemic, but serious consideration should be paid towards strategies that allow schools to remain open, particularly for children younger than ten years.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
6.
Shapiro Ben David S, Cohen D, Tasher D, Geva A, Azuri J, Ash N. COVID‐19 in children and the effect of schools reopening on potential transmission to household members. Acta Paediatr. 2021;110:2567–2573. 10.1111/apa.15962
Joseph Azuri, Nachman Ash contributed equally to this work.
Funding information
No funding was received
REFERENCES
- 1. Chen F, Liu ZS, Zhang FR, et al. First case of severe childhood novel coronavirus pneumonia in China. Chin J Pediatr. 2020;58(3):179‐183. [DOI] [PubMed] [Google Scholar]
- 2. Zimmermann P, Curtis N. Coronavirus infections in children Including COVID‐19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39(5):355‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Han MS, Choi EH, Chang SH, et al. Clinical characteristics and viral RNA detection in children with coronavirus disease 2019 in the republic of korea. JAMA Pediatr. 2020;28:e203988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chao JY, Derespina KR, Herold BC, et al. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 at a tertiary care medical center in New York City. J Pediatr. 2020;223:14‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ji LN, Chao S, Wang YJ, et al. Clinical features of pediatric patients with COVID‐19: a report of two family cluster cases. World J Pediatr. 2020;16:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li F, Li YY, Liu MJ, et al. Household transmission of SARS‐CoV‐2 and risk factors for susceptibility and infectivity in Wuhan: a retrospective observational study. Lancet Infect Dis. 2021;S1473‐3099(20):30981‐30986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ehrhardt J, Ekinci A, Krehl H, et al. Transmission of SARS‐CoV‐2 in children aged 0 to 19 years in childcare facilities and schools after their reopening in may 2020, Baden‐Württemberg, Germany. Euro Surveill. 2020;25(36):pii=2001587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Israel Ministry of Health (MoH) . COVID‐19 Guidelines. 2020. Hebrew. Available at: https://govextra.gov.il/ministry‐of‐health/corona/corona‐virus/medical‐guidelines‐corona/. Accessed 25 May 2020. [Google Scholar]
- 10. Stein‐Zamir C, Abramson N, Shoob H, et al. A large COVID‐19 outbreak in a high school 10 days after schools' reopening, Israel, May 2020. Euro Surveill. 2020;25(29):2001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. CDC Centers for Disease Control and Prevention . Demographic Trends of COVID‐19 cases and deaths in the US reported to CDC. Available at: https://covid.cdc.gov/covid‐data‐tracker/#demographics. Accessed 6 June 2021. [Google Scholar]
- 12. Statista . Age Distribution of Coronavirus (COVID‐19) Cases in South Korea as of August 21, 2020. Available at: https://www.statista.com/statistics/1102730/south‐korea‐coronavirus‐cases‐by‐age/. Accessed 25 August 2020. [Google Scholar]
- 13. OECD . OECD Family Database. Available at: http://www.oecd.org/els/soc/SF_1_4_Population_age_children_youth_dependency_ratio.pdf. Accessed 25 September, 2020.
- 14. Sola AM, David AP, Rosbe KW, Baba A, Ramirez‐Avila L, Chan DK. Prevalence of SARS‐CoV‐2 infection in children without symptoms of coronavirus disease 2019. JAMA Pediatr. 2021;175(2):198‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patel NA. Pediatric COVID‐19: Systematic review of the literature. Am J Otolaryngol. 2020;41(5):102573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poline J, Gaschignard J, Leblanc C, Madhi F, Foucaud E, Nattes E, et al. Systematic SARS‐CoV‐2 screening at hospital admission in children: a French prospective multicenter study. Clin Infect Dis. 2020(ciaa1044). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Central bureau of statistics . Publications and Media Releases. Available at: https://www.cbs.gov.il/en/mediarelease/Pages/2017/Projections‐of‐Israel‐Population‐until‐2065.aspx. Accessed 25 September, 2020. [Google Scholar]
- 18. Goyal MK, Simpson JN, Boyle MD, et al. Racial and/or ethnic and socioeconomic disparities of SARS‐CoV‐2 infection among children. Pediatrics. 2020;146(4):e2020009951. [DOI] [PubMed] [Google Scholar]
- 19. Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Household transmission of SARS‐CoV‐2: a systematic review and meta‐analysis. JAMA Netw Open. 2020;3(12):e2031756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Somekh E, Gleyzer A, Heller E, et al. The role of children in the dynamics of intra family coronavirus 2019 spread in densely populated area. Pediatr Infect Dis J. 2020;39(8):e202‐e204. [DOI] [PubMed] [Google Scholar]
- 21. Park YJ, Choe YJ, Park O, et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26(10):2465‐2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Posfay‐Barbe KM, Wagner N, Gauthey M, et al. COVID‐19 in children and the dynamics of infection in families. Pediatrics. 2020;146(2):e20201576. [DOI] [PubMed] [Google Scholar]
- 23. Zimmerman KO, Akinboyo IC, Brookhart MA, et al. Incidence and secondary transmission of SARS‐CoV‐2 infections in schools. Pediatrics. 2021;8:e2020048090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adam DC, Wu P, Wong JY, et al. Clustering and superspreading potential of SARS‐CoV‐2 infections in Hong Kong. Nat Med. 2020;26:1714‐1719. [DOI] [PubMed] [Google Scholar]
- 25. Macartney K, Quinn HE, Pillsbury AJ, et al. Transmission of SARS‐CoV‐2 in Australian educational settings: a prospective cohort study. Lancet Child Adolesc Health. 2020;S2352‐4642(20):30251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Somekh I, Shohat T, Boker LK, Simões EAF, Somekh E. Reopening schools and the dynamics of SARS‐CoV‐2 infections in israel: a nationwide study. Clin Infect Dis. 2021(ciab035). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lopez AS, Hill M, Antezano J, et al. Transmission dynamics of COVID‐19 outbreaks associated with child care facilities‐salt lake city, utah, april‐july 2020. MMWR Morb Mortal Wkly Rep. 2020;69(37):1319‐1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lachassinne E, de Pontual L , Caseris M, et al. SARS‐CoV‐2 transmission among children and staff in daycare centres during a nationwide lockdown in France: a cross‐sectional, multicentre, seroprevalence study. Lancet Child Adolesc Health. 2021;S2352‐4642(21):00024‐00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. European Centre for Disease Prevention and Control (ECDC) . Heating, Ventilation and Air‐Conditioning Systems in The Context of COVID‐19. ECDC; 2020. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/Ventilation‐in‐the‐context‐of‐COVID‐19.pdf. Accessed 15 September 2020. [Google Scholar]
- 30. Falk A, Benda A, Falk P, Steffen S, Wallace Z, Høeg TB. COVID‐19 cases and transmission in 17 K‐12 schools‐wood county, wisconsin, august 31‐november 29, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(4):136‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Israel Ministry of Health . Four cases of COVID‐19 Varient found in United Kingdom have been discovered in Israe . Ministry of Health. 2020. Available at: https://www.gov.il/en/departments/news/23122020‐07. Accessed 19 April 2021. [Google Scholar]
- 32. Davies NG, Abbott S, Barnard RC, et al. Estimated transmissibility and impact of SARS‐CoV‐2 lineage B.1.1.7 in England. Science. 2021;372(6538):eabg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]


