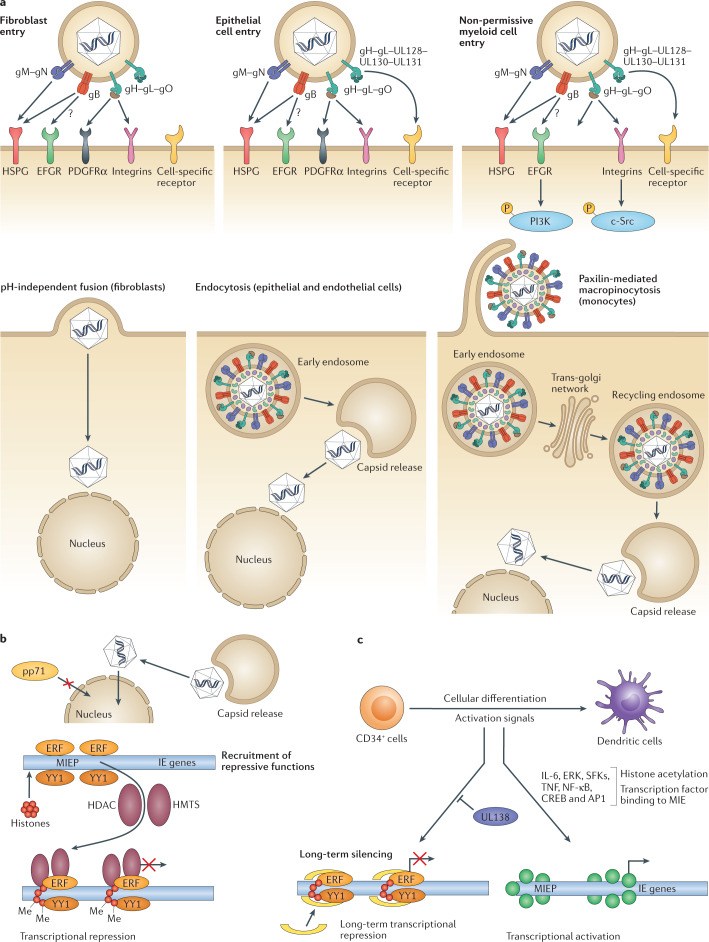
Fig. 1. Overview of human cytomegalovirus entry into target cells and the establishment of latency in non-permissive myeloid cells.
a | Human cytomegalovirus (HCMV) glycoproteins on the surface of the virion engage with receptors on the surface of cells and can drive entry by multiple processes in a cell type-dependent manner. In fibroblasts, glycoprotein H (gH), gL and gO form a trimer that binds to platelet-derived growth factor-α (PDGFRα) and co-receptors. This binding triggers gB to fuse directly with the plasma membrane at neutral pH. In permissive epithelial and endothelial cells, gH and gL form a pentameric complex with three other proteins encoded within the ULb′ region (UL128–UL130–UL131). This pentameric complex binds to neuropilin 2 and triggers pH-dependent endocytosis. The fusion activity of gB becomes relevant for escape from the endosome. For both cell types, once the capsid and associated tegument proteins are released into the cytoplasm they move independently to the nucleus, where virion DNA interacts with the nuclear pore complex to transition into the nucleus. Infection of myeloid cells (including potential sites of latency) involves macropinocytosis. In myeloid cells where HCMV establishes latency (that is, CD34+ cells), activation of epidermal growth factor receptor (EGFR) and integrin-mediated src family kinase (SFK) signalling via gB and pentamer, respectively, is required for the trafficking of HCMV DNA contained within the capsid to the nucleus via recycling endosomes. An overview of cellular receptors is provided in ref.118. b | Establishment of latency is dependent on effective silencing of major immediate early (MIE) gene expression. In CD34+ cells, this is likely a combination of host and viral-encoded events including a failure of virion transactivators (for example, pp71) to enter the nucleus coupled with a host environment of high levels of transcriptional repressors of the MIE promoter (MIEP). The result is establishment of a repressive chromatin phenotype driving MIEP silencing that is maintained by viral UL138 gene expression. c | Cellular differentiation to a dendritic cell promotes induction of transcription from the MIE locus through the activity of host chromatin remodelling enzymes. This process is responsive to inflammatory cytokine signalling (for example, tumour necrosis factor (TNF) and interleukin 6 (IL-6)) through extracellular signal-regulated kinase (ERK) and SFK signalling pathways. Binding sites for multiple transcription factors (for example, nuclear factor-κB (NF-κB), cAMP response element binding protein (CREB), activator protein 1 (AP1)) in the MIEP have been hypothesized to be important for the control of MIE gene expression upon reactivation. Part a is adapted from Murray et al.119, 2018 CC BY 4.0 (https://www.mdpi.com/2076-0817/7/1/30).