Abstract
Mango (Mangifera indica) is a tropical fruit highly desired for its vitamin content and flavor, but its peel is considered a byproduct or waste. However, mango peel contains some bioactive compounds that improve food quality matrix for the development of edible coatings or films. The effect of phenolic mango (Mangifera indica) peel extracts on the physicochemical, rheological, and microstructural properties of xanthan gum-based coating solutions was evaluated. The obtained solutions were stable during the study period and presented a non-Newtonian fluid type shear-thinning behavior described by Ostwald–de Waele. Moreover, viscoelastic properties revealed that the elastic modulus was higher than the viscous modulus, showing a characteristic of weak gels. The addition of extracts did not alter the shear rate and viscoelastic character of the solutions, preserving the pseudoplasticity and weak gel behavior of xanthan gum associated with spreadability and adherence of coatings; it modified the gel structure as a function of temperature. Furthermore, the coating solutions of xanthan gum and phenolic mango peel extracts are an alternative to develop complex food systems such as edible coatings, edible films, or delivery systems.
1. Introduction
Mango (Mangifera indica) is a fruit highly desired due to its flavor and micronutrients (vitamin E, vitamin C, calcium, selenium, potassium, magnesium, and iron)1,2 and bioactive compounds (phenolic esters, flavonoids, carotenoids, and dietary fibers),3,4 with antioxidant, antimicrobial, anti-inflammatory, or antidiabetic activity, being a potential source of natural ingredients with applications in biological fields.5,6 The interest in using natural ingredients is often associated with the opportunity to recover functional and bioactive compounds from food waste and byproducts, such as mango leaves, peel, or seeds. Various phenolic extracts have been reported in the literature, such as essential oils, ultrasound-assisted extracts, or supercritical phenolic-rich extracts, that are added into gum-based coating materials, which enhances the antioxidant and/or antimicrobial activity in films and coatings.7,8 Nevertheless, it is estimated that most of the compounds currently in development are prone to dissolution problems and can easily be degraded under high temperatures, low pH, light, and reactive oxygen species,9 making their application in food products challenging. Moreover, their use is limited because they give flavor to food, contributing to negative organoleptic characteristics,10 and they can change film properties, making it necessary to characterize the material to observe if both components presented synergistic or antagonistic properties of the films.11
There are many substances employed for the development of edible coatings, such as polysaccharides, proteins, hydrocolloids, or composite materials. Xanthan gum (XG), a natural, nontoxic, and biocompatible source, is approved by the Food and Drug Administration (FDA) as a safe polymer in the food industry; it facilitates inorganic particle adsorption and forms a stable emulsion without lowering the interfacial tension.12 Its use as a food coating material is also advantageous due to its excellent stability in thermal and acidic systems and its ability to enhance viscosity stability.13−15 However, continuous research and the introduction of novel concepts for possible modifications result in coating formulations related to the addition of bioactive compounds.
Dispersion and emulsion represent a strategy to solubilize lipophilic ingredients in aqueous media and design new products with an active component that can carry out its functionality in more complex media through the stability provided by emulsion products.16 The knowledge of rheological properties is necessary to be able to know about the stability of the matrix and to improve the design of products and facilitate the incorporation of compounds into coating solutions.8 Factors such as particle concentration, dispersed phase fraction, homogenization method, ionic strength, pH, temperature, and viscosity, depending on the concentration of dispersion,16 affect the physical stability of dispersions.
Taking into account that the addition of phenolic compounds modifies the physical and chemical properties of colloidal systems, which improves their potential application as an ingredient in the development of food products, this work aimed at the development of xanthan gum-based coating solutions enriched with phenolic mango (M. indica) peel extracts and characterization of their rheological and microstructural properties.
2. Results and Discussion
2.1. Ethanolic Ultrasound-Assisted Extracts from Mango Peel
The extraction yield for ethanolic mango peel extracts was 7.02 ± 1.14% at 15 min (MPe-15) and 11.35 ± 2.05% at 30 min (MPe-30), showing an increase in yield with an increase in the time of extraction. The samples were exposed to cavitation waves with ultrasound for a longer time in treatment processes, allowing a greater mass transfer and acceleration of the swelling and hydration of plant tissues, which results in increased extraction rates.17 Moreover, a similar trend in TPC was obtained (p > 0.05), where 262.32 ± 0.08 and 294.60 ± 0.03 mg gallic acid equivalents (GAE)/100 g of extract were obtained for MPe-15 and MPe-30, respectively. The results may be attributed to the fact that the extraction was governed by the solvent polarity and the synergistic interaction between them.18 Ethanol is classified as a polar protic solvent, due to its hydroxyl groups, and a hydrogen bond donor, resulting in preferential extraction of low-molecular-weight compounds, such as phenolic compounds. Thus, they have an affinity with solid matrix bioactive compounds, making the solvent system selective in the extraction. Mango peel comprises specific compounds such as polyphenols, anthocyanins, carotenoids, flavanols, vitamin E, and vitamin C.18
The total phenolic content (TPC) reported in our study was different from that reported by other authors: Haden, 293 mg/100 g of DW;19 and Kent, 234–9121 mg/100 g of DW.20 Using dry mango peel and an ethanol–acetone blend extract (60–40%), TPC obtained was 205.08 mg GAE/100 g of dry matter,18 and on microwave-assisted extraction with a 70% aqueous ethanol extract (50 °C), TPC obtained was 723.2 ± 0.93 mg GAE/kg of dry mango peel.21 These differences could be attributed to different factors such as the genetic basis, agronomic practices, harvest stage, and environmental conditions, among others.22,23 Therefore, higher recovery of TPC was observed in mango peel MPe-30, thereby considering this an appropriate sample to develop a xanthan gum-based coating solution.
2.2. Coating Solutions Enriched with Phenolic Mango Peel Extracts (MPe)
Eight coating solutions were prepared to evaluate the percentage of XG and MPe. The dispersions formulated did not show phase separation. The solutions were stable during the 8-day storage period at 25 °C, influenced by the amount of XG and Tween 80, which acted as a stabilizer24 and surfactant, thereby helping to reduce the interfacial tension between the phases stabilizing the solutions.25 MPe was dispersed in an aqueous medium and remained stable, presenting attractive characteristics for use in edible coatings,7,8 to reduce oxidation reactions and increase the shelf life of some fresh products.26 It has been observed that variations in the concentration of raw materials significantly affect the physical properties of the coating solutions. The addition of MPe decreases the pH values of coating solutions and increases their soluble solids (°Brix) in comparison with xanthan gum-based coating solutions (p < 0.05) (Table 1). The physicochemical properties of solutions were influenced by the components; this is due to bonds between the hydrophilic groups of polysaccharides and the solvent, such as water.27 The total phenolic contents of XG coating solutions were 1.76 ± 0.31 and 3.40 ± 0.94 mg GAE/100 g of solutions for samples of 0.5 and 1.0% XG. Then, as was expected, the addition of MPe resulted in a linear increase of TPC in all cases, samples with 0.5% XG (R2 = 0.997) and 1.0% XG (R2 = 0.998).
Table 1. Physicochemical Properties of Coating Solutions Enriched with Phenolic Mango Peel Extractsa.
| sample code | pH | °Brix | mg GAE/100 g of solutions |
|---|---|---|---|
| X1 | 5.25 ± 0.01b | 1.81 ± 0.14a | 3.40 ± 0.94a |
| MPe-X2 | 4.19 ± 0.04c | 2.85 ± 0.07b | 17.36 ± 2.20b |
| MPe-X3 | 4.01 ± 0.01ac | 3.82 ± 0.14bc | 28.81 ± 0.31c |
| MPe-X4 | 3.88 ± 0.01a | 4.05 ± 0.07c | 43.21 ± 1.76d |
| X5 | 5.50 ± 0.01b | 1.34 ± 0.14a | 1.76 ± 0.31a |
| MPe-X6 | 3.99 ± 0.03a | 2.45 ± 0.07b | 15.60 ± 1.20b |
| MPe-X7 | 3.99 ± 0.03a | 3.15 ± 0.21b | 31.14 ± 0.25c |
| MPe-X8 | 3.85 ± 0.08a | 3.63 ± 0.14bc | 42.71 ± 3.14d |
Data are the mean ± standard deviation. Different letters in the same column symbolize a statistically significant difference (p < 0.05).
2.3. Color Parameters of Coating Solutions Enriched with Phenolic MPe
Color parameters of the coating solutions are summarized in Table 2. The lightness (L*) of the samples decreases significantly (p < 0.05) with MPe, while the color saturation (p < 0.05), represented by chroma (C*), increases. Xanthan gum-based solutions were more visually transparent than samples with MPe. Color parameters a* (+, red; −, green) and b* (+, yellow; −, blue) are good indicators of film color; coating solutions with MPe presented a green color related to the negative values of a* (greenness) in contrast to the positive value with the xanthan gum-based solution.
Table 2. Color Parameters of Coating Solutions Enriched with Phenolic Mango Peel Extractsa.
| sample code | L* | a* | b* | C* | ΔE |
|---|---|---|---|---|---|
| X1 | 57.43 ± 4.94d | 0.12 ± 0.01a | 4.47 ± 0.35a | 6.14 ± 0.16a | |
| MPe-X2 | 41.11 ± 1.15bc | –2.26 ± 0.08b | 19.87 ± 0.32c | 20.02 ± 0.43c | 254.31 ± 0.14a |
| MPe-X3 | 33.07 ± 4.24b | –2.75 ± 0.39b | 15.31 ± 4.91b | 15.59 ± 4.75b | 370.48 ± 63.77b |
| MPe-X4 | 26.55 ± 0.85a | –4.16 ± 0.30c | 13.48 ± 0.54b | 13.58 ± 0.56b | 528.14 ± 18.79c |
| X5 | 49.46 ± 6.80c | 0.71 ± 0.01a | 2.92 ± 0.54a | 5.54 ± 0.35a | |
| MPe-X6 | 33.21 ± 4.42b | –2.37 ± 0.53b | 16.32 ± 0.31b | 16.50 ± 0.37b | 231.17 ± 63.45a |
| MPe-X7 | 39.08 ± 2.59bc | –2.55 ±1.85b | 22.76 ± 1.18d | 22.94 ± 1.29c | 252.77 ± 6.79a |
| MPe-X8 | 47.77 ± 1.16c | –3.54 ± 0.34c | 25.34 ± 0.43d | 25.58 ± 0.46c | 253.91± 7.87a |
Data are the mean ± standard deviation. Different letters in the same column symbolize a statistically significant difference (p < 0.05).
As regards the color aspect, color parameters and the total color difference (ΔE) were evaluated. ΔE values increase with the percentage of MPe. Therefore, the greenish shade of the coating solutions became more intense upon increasing the concentration of MPe, whereby this trend was replicated in their visual appearance.
2.4. Rheological Characterization
2.4.1. Steady-State Viscous Flow
Rheological properties present important considerations for developing edible coatings,28 related to the guarantee of product homogeneity and redispersion of ingredients. The potential properties of the materials developed as food coatings, such as their spreadability, viscosity, and stability against oscillatory tests, should be evaluated.28 However, only limited research has been conducted to investigate phenolic extract interactions on the physicochemical properties of polymeric systems, considering that phenolic compounds can change the rheological gelling properties.29 Moreover, there are many variables in the development of food coating materials; it was considered that a rheological study would be of great value to analyze the main factors influencing the percentage of XG and the concentration of natural extracts employed in the polymeric system.
The viscous curve of coating solutions at 25 °C is shown in Figure 1. A decrease of viscosity (η) with the increase of shear rate (γ̇) was observed, which is characteristic of a non-Newtonian fluid type shear-thinning behavior,30 attributed to the content of XG.31 This can be explained by the structural deformation of the network formed in the equilibrium state. With the increase of shear rate, the particles are aligned in the direction of flow and the reticular structure of the polysaccharide molecules is broken and hence the resistance to flow is lost.32 These findings are in concordance with those of Kumar andMandal33 and Matos et al.,34 indicating that colloidal dispersions present shear-thinning behavior. Other studies that employed phytochemicals to develop dispersions showed similar results: Silva-Weiss35 prepared polyphenol-rich murta leaf extracts in hydrocolloid blends; Zhang et al.36 prepared chitosan/zein edible films with the addition of α-tocopherol; and Tian et al.37 developed water-in-oil-in-water emulsions by adding a xanthan gum–locust bean gum mixture to encapsulate tea polyphenols. All authors obtained dispersions with shear-thinning behavior, explained by the network structural deformation in the equilibrium state.
Figure 1.
Viscosity η (Pa·s) vs shear rate (1/s) and fitting of flow curves with the Ostwald–de Waele model of xanthan gum-based coating solutions enriched with MPe at 25 °C.
Consequently, the variation of viscosity as a function of shear rate was adjusted using the Ostwald–de Waele model, represented by eq 1, obtaining R2 > 0.998
| 1 |
where η is the apparent viscosity, k is the consistency index, and n is the flux index. The rheological parameter results are presented in Table 3, which confirm the shear-thinning behavior of solutions presenting a flux index of n < 116 when the percentage of extracts and gum does not vary (p > 0.05). The consistency index (k) increases significantly (p < 0.05) with XG percentage, as expected, due to the ability to increase the viscosity at a higher concentration of gum.25 The k value increases significantly with the concentration of extract in samples with 1% XG, samples with 0.5% being an exception. Moreover, the consistency index increases according to the solid content and the concentration of the dispersed phase38 due to the higher content of polysaccharides present in them.39 A similar behavior has been reported by Cofelice et al.16 and Baéz et al.,40 where the rubber concentration as a surfactant influenced the flow parameters against the bioactive component effect. Likewise, the concentration of phenolic extracts did not change the dispersion flow behavior; similar findings were reported by Vuillemin et al.41 in solutions of Arabic gum with ferulic acid, contrary to what was reported for pectin29 and chitosan.8,42 However, despite the presence of phenolic compounds in XG, its rheological behavior did not change. At the molecular level, weak associations between the polymeric chains of XG, leading to facilitated flow upon shearing, favors shear-thinning behavior.43
Table 3. Adjustment Parameters of Xanthan Gum-Based Coating Solutions Enriched with Phenolic Mango (M. indica) Peel Extracts Using the Ostwald–de Waele Modela.
| sample code | k | n | R2 |
|---|---|---|---|
| X1 | 15.78 ± 0.06 a | 0.14 ± 0.01a | 0.99 |
| MPe-X2 | 18.34 ± 1.13b | 0.13 ± 0.01a | 0.99 |
| MPe-X3 | 18.35 ± 0.25b | 0.17 ± 0.04a | 0.99 |
| MPe-X4 | 19.14 ± 0.66b | 0.13 ± 0.01a | 0.99 |
| X5 | 4.73 ± 0.01c | 0.18 ± 0.01a | 0.99 |
| MPe-X6 | 5.11 ± 0.01c | 0.15 ± 0.01a | 0.99 |
| MPe-X7 | 5.33 ± 0.54c | 0.17 ± 0.01a | 0.99 |
| MPe-X8 | 4.53 ± 0.13c | 0.19 ± 0.02a | 0.99 |
Data are the mean ± standard deviation. Different letters in the same column symbolize a statistically significant difference (p < 0.05).
2.4.2. Viscoelastic Properties
Figure 2 shows the viscoelastic properties of coating solutions in the linear viscoelastic range. The storage modulus G′ was greater than the loss modulus G″ in all frequency ranges studied (Figure 2a). The elastic component of the dispersions predominates over the viscous component,44 characteristic of a gel solution network macromolecule entangled in all dispersions. The G′ and G″ values indicate a high dependency on the gum percentage function, where the samples containing 1% XG are stronger than the samples with 0.5% XG. All samples developed a plateau zone showing a slight dependency, quasi-elastic behavior, typical of systems known as weak gels.45 Nevertheless, the MPe addition did not present representative changes in the behavior, which means that the ethanolic extract of mango peel could be employed in a high proportion (3%) and would not modify the viscoelastic properties of samples. Similar results were reported in lemongrass essential oil16 and whey protein isolate and xanthan gum gels with curcumin.46
Figure 2.

Viscoelastic properties of xanthan gum-based coating solutions enriched with phenolic mango (M. indica) peel extracts at 25 °C. (a) Frequency sweep module G′ and G″ (Pa). (b) Loss tangent Tan δ and (c) complex viscosity |η*| (Pa·s) vs angular frequency (ω) (rad/s).
To analyze the effect of the addition of mango peel extracts and the concentration of XG, the G′ and G″ as a function of frequency were fitted to the power law using eqs 2 and 3.47
| 2 |
| 3 |
The values of the parameters (k′, n′, k″, and n″) for each solution are listed in Table 4. XG concentrations had a significant influence on the consistency index (k′ and k″) of the dispersions (p < 0.05), where samples with 1% gum presented the highest values, but the extract also had an influence on the consistency index (p < 0.05), with an increase of k′ and k″ in samples with 0.5% gum. Due to an increase in particles per unit volume, the spaces between particles decreased, leading to an increase in consistency,48 but a decrease was observed in samples with 1% gum.
Table 4. Viscoelastic Parameters of Xanthan Gum-Based Coating Solutions Enriched with Phenolic Mango (M. indica) Peel Extracts at 25 °C, Adjusted to the Power-Law Modela.
| sample code | k′ (Pa·sn) | n′ | R2 | k″ (Pa·sn) | n″ | R2 | Tan(δ) |
|---|---|---|---|---|---|---|---|
| X1 | 55.87 ± 1.41a | 1.23 ± 0.01a | 0.94 | 12.83 ± 0.16a | 1.19 ± 0.01a | 0.97 | 0.19a |
| MPe-X2 | 56.68 ± 0.8a | 1.16 ± 0.01b | 0.95 | 12.47 ± 0.05b | 1.11 ± 0.01b | 0.98 | 0.18a |
| MPe-X3 | 52.96 ± 0.79b | 1.13 ± 0.01c | 0.92 | 11.59 ± 0.05c | 1.09 ± 0.01b | 0.97 | 0.18a |
| MPe-X4 | 49.49 ± 0.72c | 1.14 ± 0.01c | 0.93 | 10.84 ± 0.06d | 1.10 ± 0.01b | 0.96 | 0.17a |
| X5 | 7.70 ± 0.25d | 1.18 ± 0.01d | 0.93 | 2.26 ± 0.28e | 1.32 ± 0.03c | 0.78 | 0.32bc |
| MPe-X6 | 10.57 ± 0.20e | 1.17 ± 0.01bd | 0.93 | 2.20 ± 0.03e | 1.15 ± 0.01d | 0.96 | 0.27b |
| MPe-X7 | 10.17 ± 0.21e | 1.18 ± 0.01d | 0.93 | 3.31 ± 0.04f | 1.14 ± 0.01d | 0.95 | 0.30b |
| MPe-X8 | 12.29 ± 0.26f | 1.16 ± 0.01b | 0.91 | 3.55 ± 0.02g | 1.14 ± 0.01d | 0.98 | 0.28b |
Data are the mean ± standard deviation. Different letters in the same column symbolize a statistically significant difference (p < 0.05).
The addition of extracts presented a slight decrease in n′ and n″ at the same time and the addition of gum increased it. In all cases, their values were consistently higher than 1 and remained in the range of 1.10–1.32, where the solutions with mango peel extracts presented the highest values; this means that the addition of mango peel extracts resulted in lower dependency on the frequency of dispersions.48 This may be explained by the increase in interactions in their internal networks, primarily physical, thus suggesting a stronger viscoelastic character for solutions with mango peel extracts.49
The loss factor (Tan δ = G″/G′) analyzed against the angular frequency was used to evaluate the liquid or solid behavior (purely elastic, δ = 0° and G′ > G″; purely viscous, δ = 90° and G″ > G′).50Figure 2b shows that tangent values of δ < 1 were observed for all solutions, which reflects that storage modulus G′ was always greater than G″, thus confirming the elastic behavior of the solutions and following the classification according to the value of the tangent. Due to the concentration of gum, the curves with 1% XG were closer to 0, presenting a more elastic nature. The addition of XG in the internal water phase probably enhanced the viscoelastic properties; the extract–water interface complex viscosity |η*| is shown in Figure 2c. The inverse potential dependency of the complex viscosity on the angular frequency (ω) was confirmed. Although the samples with the highest gum concentration exhibited higher viscosity values, all samples presented a thinning behavior with an increase in ω.
To evaluate the behavior of the solution as a function of temperature, a ramp of temperature was carried out at a heating rate of 5 °C/min in the linear viscoelastic region (LVR) and 1 Hz frequency. The effect of temperature on G′ and G″ values of different coating solutions is presented in Figure 3. The solutions were prepared with 1% XG (Figure 3a). G′ was higher than G″ at all temperatures and it did not exhibit any cross-over between the moduli. From 20 to 65 °C, all solutions maintained their behavior, after which G′ and G″ increased as temperature increased; G′ and G″ did not intersect at any point, which is associated with a gel–sol behavior as a function of temperature. Therefore, in this case, the melting point does not exist; thus, melting temperature was not found. Coating samples prepared with 1% XG did not present thermoreversibility characteristics. In all samples, G′ and G″ increased with an increase in the temperature, which probably related to the formation of hydrophobic linkages within XG molecules, confirming the reinforcement of elastic properties with an increase in temperature.51 It is known that thermoreversibility phenomena are present when hydrogen bonds maintain the XG network structure, mainly by the interaction between charges from water molecules and polysaccharides. Once the system is heated, absorbed energy causes water molecules to move faster, the pectin gel structure is lost, and, consequently, it returns to the dispersion state.52
Figure 3.

Temperature sweep ramp of xanthan gum-based coating solutions enriched with phenolic mango (M. indica) peel extracts at 25 °C. (a) Samples prepared with 1% xanthan gum and (b) 0.5% xanthan gum.
Figure 3b presents the behavior of G′ and G″ as a function of temperature for samples prepared with 0.5% XG. In this case, for X5 (xanthan gum-based coating solution) and MPe-X6, G′ was higher than G″ and did not present thermoreversibility characteristics. Different behaviors were observed in the MPe-X7 and MPe-X8 solutions with 2 and 3% MPe, respectively. For MPe-X7, G′ and G″ presented similar values until the temperature reached 50 °C; at this point, an increase of modulus was observed where G′ was higher than G″. In this type of structure, water is trapped inside, providing volume and plasticity. When the system is heated, water molecules break away and gel volume decreases significantly due to water loss and an increment of G′ and G″ magnitudes is observed.52 For MPe-X8, G′ was closer to G″ and the modulus decreased, which is connected with increasing fluidity; this weakens the polymer structure network due to the change from a helical structure to a random coil at higher temperatures.53
2.4.3. Determination of the Cox–Merz Rule
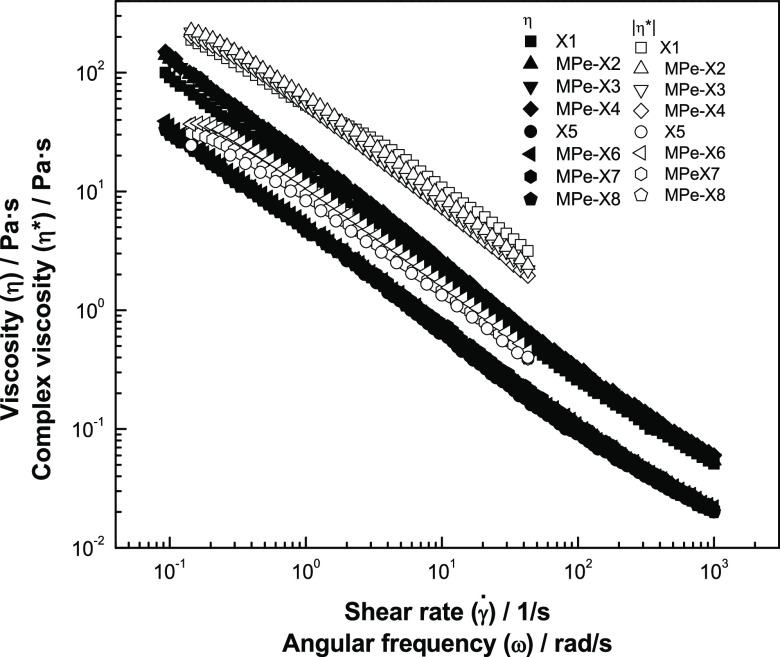
The Cox–Merz law (eq 4) relates the oscillatory and stationary shear properties of solutions, comparing the apparent viscosity (η) and the complex viscosity |η*| at equal values of deformation speed and angular frequency for complex food systems, solutions, and different polymers.54
| 4 |
To verify that the Cox–Merz law was applicable in xanthan gum-based coating solutions enriched with phenolic mango peel extracts, the curves for apparent viscosity and complex viscosity vs deformation speed and angular frequency were compared as shown in Figure 4. Complex viscosity values were higher than those of the apparent viscosity in all solutions, which indicates that the Cox–Merz law did not fit for standardized coating solutions. The nonapplicability of this law is due to the formation of aggregate structures in solutions,55 which is attributed to the addition of high-viscosity extracts. Solutions that normally present graphs with |η*| > η are from weak gels, and the difference between them increases in proportion to the weak nature of gels.56
Figure 4.
Comparison of complex viscosity |η*| and apparent viscosity (η) (Pa·s) vs angular frequency (rad/s) and strain rate (1/s) of xanthan gum-based coating solutions enriched with phenolic mango (M. indica) peel extracts at 25 ° C.
2.5. Microstructural Properties
To characterize the microstructural properties of coating solutions, from their morphological changes to the influence of mango peel extracts, the samples were analyzed by optical microscopy. On adding mango peel extracts, apparent dropletlike spots were observed, indicating the successful incorporation of the extracts into xanthan gum-based solutions. Microstructures of dispersions were nonuniform, and it was possible to visualize the spots uniformly distributed, promoting a structural discontinuity. Larger and spherical extract structures of different sizes and high porosity were seen in micrographs, presenting droplet sizes of less than 100 μm in all cases (Figure 5). The samples containing XG, due to their larger molecular weight and high viscosity, which increased each viscosity phase, hindered the movement of dispersed droplets and reduced their coalescence rate.57
Figure 5.
Micrographs of stabilized xanthan gum-based coating solutions enriched with phenolic mango (M. indica) peel extracts at 25 °C: (a) MPe-X2, (b) MPe-X3, (c) MPe-X4, (d) MPe-X6, (e) MPe-X7, and (f) MPe-X8.
The samples presented a different distribution of the dispersed phase according to their mango peel extract concentration. The drops of the extract were more dispersed at lower concentrations. At higher concentrations, the drops started to form aggregates and the extract covered a larger interparticle area, thereby increasing the interactions between particles and thus altering the rheological properties depending on the percentage of xanthan gum.48 As mentioned earlier, the mango peel extract dispersion presented overall stability, and the use of XG enhanced the stability of dispersions against coalescence and phase separation.
2.6. Potential Applications of XG-Based Coating Solutions Enriched with Phenolic MPe
Xanthan gum has attracted scientific interest due to its solubility, rapid hydration, water-binding properties, thermal pH, and salt stability.58 Moreover, it possesses interesting rheological properties, which makes it a good material base for the development of coatings; the effect of XG coatings has been studied in freshly cut pears,59 minimally processed prickly pears, pumpkins,60 and freshly cut apples.61 The addition of phenolic MPe as a potential ingredient has been employed in developing coating solutions as an alternative in the different studied applications of XG, e.g., edible coating, films, and as a carrier of bioactive compounds, due to their technological and rheological properties.
Rheological properties of coating solutions, such as spreadability, thickness, uniformity of the liquid coating layer, and film performance, can be significantly affected by the type and composition of the coating constituent.62 Solutions with high viscosity retained air bubbles in the casting process; nevertheless, solutions with low viscosity facilitated its spreading on the plate where the films were formed.63 The viscous properties obtained in this work indicate that the coating solutions could be employed for edible coatings and films. For example, samples prepared with 0.5% XG presented the lowest consistency and flux indexes (Table 4), with potential use as films in the casting process, taking into account that solutions with high viscosity and low surface tension promote a better film-forming surface,64 while coatings with 1% XG could be employed for edible coatings with a dipping process. In general, a reduction in the viscosity of solutions (Figure 1) provides a processing advantage during high-shear processing operations, whereas high apparent viscosity at low-shear rates provides a better application by dipping.62 Adhesiveness of coating solutions to the covered materials during application strongly depends on the viscosity and surface tension of the coating solution.
The study of dynamic viscoelastic properties of coating solutions led to obtaining information about molecular entanglement and molecular network formation during drying.65 All studied cases presented G′ > G″ (Figure 2), indicating that the solutions with XG and MPe are valuable materials to be applied as films in food coatings since they can enhance the adhesiveness and hardness of the coating solution.
3. Materials and Methods
3.1. Plant Sample Collection and Preparation
Mango (M. indica) fruits var. fachir were purchased from the food supply center of Cartagena, Colombia, in a commercial state of maturity. The peel was removed and lyophilized using Labconco Freezone 1.5 Liter Benchtop Freeze Dry equipment. After that, dry peels were ground in a mill (IKA MF 10,2, Germany) to obtain a powder with a particle size of less than 250 μm.
3.2. Chemicals and Reagents
Ethanol (99.5% purity) was purchased from Panreac. Sodium carbonate anhydrous (99.5% purity), gallic acid standard (>98% purity), and Folin–Ciocalteu reagent were purchased from Sigma-Aldrich (St. Louis, MO). Xanthan gum (XG) was purchased from Tecnas S.A. (Colombia).
3.3. Ultrasound-Assisted Extraction of Mango Peel Byproducts
Ultrasound-assisted extraction (UAE) was carried out following the procedure described by Cofelice et al.16 by employing an ultrasonic bath with 25 kHz and an input power of 200 W. Milled mango peel was mixed with ethanol solution in a ratio of 1:10 at 25 °C for 15 (MPe-15) and 30 min (MPe-30). After completing the UAE experiments, the mixture of liquids and solids was separated by filtration and rotary evaporated. The extraction yields were calculated employing eq 5
| 5 |
3.4. Determination of Total Phenolic Contents
The total phenolic content (TPC) in mango peel extracts (MPe) was determined using the Folin–Ciocalteu method.66 Briefly, 50 μL of the extract was mixed with 3 mL of distilled water and 250 μL of Folin–Ciocalteu reagent. The content was thoroughly mixed, and after 3 min, 750 μL of sodium carbonate solution (20% mass) and 950 μL of distilled water were added to the mixture. After 2 h at room temperature and under darkness, the absorbance was measured at 760 nm using a Genesys 10S UV–vis spectrophotometer (Thermo Fischer Scientific Inc., MA). The results were expressed as GAE (mg of gallic acid equivalents/g of extract). All analyses were performed in triplicate.
3.5. Preparation of Xanthan Gum-Based Coating Enriched with M. indica Peel Extracts (MPe)
Edible coating solutions were prepared using xanthan gum (0.5 and 1% w/v) as a base material and the addition of different amounts (1, 2, and 3% w/v) of M. indica peel extracts (MPe) (Table 5). Xanthan gum solutions were dissolved in 100 mL of distilled water at neutral pH under magnetic stirring. After complete dissolution, Tween 80 (0.1% w/v in all samples) and mango peel extracts were added to a continuous phase and homogenized using a T20 digital ULTRA-TURRAX at 10 000 rpm for 7 min. The samples were centrifuged for 10 min to eliminate bubbles and obtain a homogeneous coating solution.
Table 5. Sample Code Formulations for Xanthan Gum-Based Coating Solutions Enriched with Phenolic Mango (M. indica) Peel Extracts (MPe).
| sample code | gum (%) | extract (%) |
|---|---|---|
| X1 | 1 | 0 |
| MPe-X2 | 1 | 1 |
| MPe-X3 | 1 | 2 |
| MPe-X4 | 1 | 3 |
| X5 | 0.5 | 0 |
| MPe-X6 | 0.5 | 1 |
| MPe-X7 | 0.5 | 2 |
| MPe-X8 | 0.5 | 3 |
3.6. Physicochemical Properties of Coating Solutions
The pH and soluble solid content of the coating solutions were measured following the procedures described in the AOAC method,67 and the total phenolic contents were determined by the Folin–Ciocalteu method.66 The color of the coating solution was measured with a colorimeter. Values of lightness (L*), red chromaticity (a*), and blue-yellow chromaticity (b*) were recorded to calculate the chromaticity (C*) and change in color (ΔE), based on the following equations
| 6 |
| 7 |
3.7. Rheological Characterization of Coating Solutions
The rheological characterization of coating solutions without shear history was carried out in a controlled-stress rheometer (Modular Advanced Rheometer System Haake Mars 60, Thermo-Scientific, Germany) following the procedures described by Quintana et al.8,68 using serrated plate–plate geometry to prevent slip effects. The temperature was fixed at 25 °C (using a Peltier system), and each sample was equilibrated in 600 s before the rheological test to ensure the same recent thermal and mechanical history for each sample.
Viscous flow tests were done at a steady state, analyzing the variation of viscosity in a range of deformation rates between 10–3 and 103 s–1. Small-amplitude oscillatory shear (SAOS) tests were performed to obtain the viscoelastic responses. In this way, stress sweeps were carried out at a frequency of 1 Hz, applying an ascending series of stress values from 10–3 and 103 Pa to determine the linear viscoelasticity interval. Frequency sweeps were performed to obtain the mechanical spectrum by applying a stress value within the linear viscoelastic range in a frequency range between 10–2 and 102 rad/s. Ramping of temperature was done from 20 to 80 °C, under constant frequency (1 Hz) in the LVR and at a heating rate of 5 °C/min. All analyses were carried out in duplicate.
3.8. Microstructural Characterization
The samples were taken after their preparation and were observed in a Carl Zeiss Primo Star microscope with a 100× objective to know the internal distribution of dispersions with different percentages of MPe.
3.9. Statistical Analysis
Statistical analysis of the results was performed using Statgraphics Centurion XVI (Statgraphics, Rockville, MD). An analysis of variance (ANOVA) (unidirectional) test was applied to determine statistically significant differences (p < 0.05) between samples submitted to characterizations.
4. Conclusions
Ethanolic extracts of mango peel var. fachir with a high total phenolic content were dispersed effectively in a xanthan gum-based coating solution. The samples can be stored for 8 days without destabilization or phase separation. The addition of MPe presents a decrease in pH and an increase in total soluble solid contents. The TPC of coating solutions is proportional to the percentage of extracts added and contributes to solutions. The rheological characterization of solutions presents the behavior of a non-Newtonian fluid of shear-thinning type in all cases and can be described by the Ostwald–de Waele model obtaining an average value of R2 ≥ 0.998 and with a viscoelastic behavior as a solid rather than as a liquid since G′ was greater than G″ in all cases that were observed. Also, the study of temperature confirms the gel–sol behavior, and hence the melting point does not exist due to the lack of cross-over between the moduli. The microstructure of coating solutions presented uniformly distributed spots, promoting a structural discontinuity; the particles were larger and spherical with a particle size of less than 100 μm. Mango peel extracts presented a good blend and stable entanglement in the gum-based coating solution and did not cause significant modifying effects on its rheological properties; therefore, MPe is a new alternative and can be used in food matrixes without causing a change in the physicochemical and sensory parameters related to rheology.
Acknowledgments
This work is part of a research program “Programa Nacional de Ciencia, Tecnología e Innovación en Ciencias Agropecuarias; Project 368-2019 code 110780864755” sponsored by MinCiencias (Colombia). The authors are grateful for their financial support.
The authors declare no competing financial interest.
References
- Meena N. K.; Asrey R. Tree Age Affects Postharvest Attributes and Mineral Content in Amrapali Mango (Mangifera indica) Fruits. Hortic. Plant J. 2018, 4, 55–61. 10.1016/j.hpj.2018.01.005. [DOI] [Google Scholar]
- Patiño-Rodríguez O.; Bello-Pérez L. A.; Agama-Acevedo E.; Pacheco-Vargas G. Pulp and Peel of Unripe Stenospermocarpic Mango (Mangifera indica L. Cv Ataulfo) as an Alternative Source of Starch, Polyphenols and Dietary Fibre. Food Res. Int. 2020, 138, 109719 10.1016/j.foodres.2020.109719. [DOI] [PubMed] [Google Scholar]
- Jahurul M. H. A.; Zaidul I. S. M.; Ghafoor K.; Al-Juhaimi F. Y.; Nyam K. L.; Norulaini N. A. N.; Sahena F.; Mohd Omar A. K. Mango (Mangifera indica L.) by-Products and Their Valuable Components: A Review. Food Chem. 2015, 183, 173–180. 10.1016/j.foodchem.2015.03.046. [DOI] [PubMed] [Google Scholar]
- Kim H.; Moon J. Y.; Kim H.; Lee D. S.; Cho M.; Choi H. K.; Kim Y. S.; Mosaddik A.; Cho S. K. Antioxidant and Antiproliferative Activities of Mango (Mangifera indica L.) Flesh and Peel. Food Chem. 2010, 121, 429–436. 10.1016/j.foodchem.2009.12.060. [DOI] [Google Scholar]
- Roleira F. M. F.; Varela C. L.; Costa S. C.; Tavares-da-Silva E. J.. Chapter 4 - Phenolic Derivatives From Medicinal Herbs and Plant Extracts: Anticancer Effects and Synthetic Approaches to Modulate Biological Activity. In Studies in Natural Products Chemistry; Atta-ur-Rahman B. T., Eds.; Elsevier, 2018; Vol. 57, pp 115–156. [Google Scholar]
- Chandrasekara A.Phenolic Acids. In Encyclopedia of Food Chemistry; Melton L.; Shahidi F.; Varelis P. B. T., Eds.; Academic Press: Oxford, 2019; pp 535–545. [Google Scholar]
- Quintana S. E.; Llalla O.; García-Risco M. R.; Fornari T. Comparison between Essential Oils and Supercritical Extracts into Chitosan-Based Edible Coatings on Strawberry Quality during Cold Storage. J. Supercrit. Fluids 2021, 171, 105198 10.1016/j.supflu.2021.105198. [DOI] [Google Scholar]
- Quintana S. E.; Llalla O.; García-Zapateiro L. A.; García-Risco M. R.; Fornari T. Preparation and Characterization of Licorice-Chitosan Coatings for Postharvest Treatment of Fresh Strawberries. Appl. Sci. 2020, 10, 8431 10.3390/app10238431. [DOI] [Google Scholar]
- Boon C. S.; McClements D. J.; Weiss J.; Decker E. A. Factors Influencing the Chemical Stability of Carotenoids in Foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. 10.1080/10408390802565889. [DOI] [PubMed] [Google Scholar]
- Heras-Mozos R.; Muriel-Galet V.; López-Carballo G.; Catalá R.; Hernández-Muñoz P.; Gavara R. Development and Optimization of Antifungal Packaging for Sliced Pan Loaf Based on Garlic as Active Agent and Bread Aroma as Aroma Corrector. Int. J. Food Microbiol. 2019, 290, 42–48. 10.1016/j.ijfoodmicro.2018.09.024. [DOI] [PubMed] [Google Scholar]
- Silva-Weiss A.; Ihl M.; Sobral P. J. A.; Gómez-Guillén M. C.; Bifani V. Natural Additives in Bioactive Edible Films and Coatings: Functionality and Applications in Foods. Food Eng. Rev. 2013, 200–216. 10.1007/s12393-013-9072-5. [DOI] [Google Scholar]
- Xu W.; Jin W.; Huang K.; Huang L.; Lou Y.; Li J.; Liu X.; Li B. Interfacial and Emulsion Stabilized Behavior of Lysozyme/Xanthan Gum Nanoparticles. Int. J. Biol. Macromol. 2018, 117, 280–286. 10.1016/j.ijbiomac.2018.05.187. [DOI] [PubMed] [Google Scholar]
- Wang C. S.; Natale G.; Virgilio N.; Heuzey M. C. Synergistic Gelation of Gelatin B with Xanthan Gum. Food Hydrocolloids 2016, 60, 374–383. 10.1016/j.foodhyd.2016.03.043. [DOI] [Google Scholar]
- Salehi F. Edible Coating of Fruits and Vegetables Using Natural Gums: A Review. Int. J. Fruit Sci. 2020, 20, S570–S589. 10.1080/15538362.2020.1746730. [DOI] [Google Scholar]
- Salehi F. Effect of Coatings Made by New Hydrocolloids on the Oil Uptake during Deep-Fat Frying: A Review. J. Food Process. Preserv. 2020, 44, e14879 10.1111/jfpp.14879. [DOI] [Google Scholar]
- Cofelice M.; Cuomo F.; Lopez F. Rheological Properties of Alginate–Essential Oil Nanodispersions. Colloids Interfaces 2018, 2, 48 10.3390/colloids2040048. [DOI] [Google Scholar]
- Gharibzahedi S. M. T.; Smith B.; Guo Y. Ultrasound-Microwave Assisted Extraction of Pectin from Fig (Ficus carica L.) Skin: Optimization, Characterization and Bioactivity. Carbohydr. Polym. 2019, 222, 114992 10.1016/j.carbpol.2019.114992. [DOI] [PubMed] [Google Scholar]
- Martínez-Ramos T.; Benedito-Fort J.; Watson N. J.; Ruiz-López I. I.; Che-Galicia G.; Corona-Jiménez E. Effect of Solvent Composition and Its Interaction with Ultrasonic Energy on the Ultrasound-Assisted Extraction of Phenolic Compounds from Mango Peels (Mangifera indica L.). Food Bioprod. Process 2020, 122, 41–54. 10.1016/j.fbp.2020.03.011. [DOI] [Google Scholar]
- Barreto J. C.; Trevisan M. T. S.; Hull W. E.; Erben G.; De Brito E. S.; Pfundstein B.; Würtele G.; Spiegelhalder B.; Owen R. W. Characterization and Quantitation of Polyphenolic Compounds in Bark, Kernel, Leaves, and Peel of Mango (Mangifera indica L.). J. Agric. Food Chem. 2008, 56, 5599–5610. 10.1021/jf800738r. [DOI] [PubMed] [Google Scholar]
- Berardini N.; Fezer R.; Conrad J.; Beifuss U.; Carle R.; Schieber A. Screening of Mango (Mangifera indica L.) Cultivars for Their Contents of Flavonol O- and Xanthone C-Glycosides, Anthocyanins, and Pectin. J. Agric. Food Chem. 2005, 53, 1563–1570. 10.1021/jf0484069. [DOI] [PubMed] [Google Scholar]
- Bai X.; Lai T.; Zhou T.; Li Y.; Li X.; Zhang H. In Vitro Antioxidant Activities of Phenols and Oleanolic Acid from Mango Peel and Their Cytotoxic Effect on A549 Cell Line. Molecules 2018, 23, 1395 10.3390/molecules23061395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson J. T.; Monteith G. R.; Roberts-Thomson S. J.; Dietzgen R. G.; Gidley M. J.; Shaw P. N. Phytochemical Extraction, Characterisation and Comparative Distribution across Four Mango (Mangifera indica L.) Fruit Varieties. Food Chem. 2014, 149, 253–263. 10.1016/j.foodchem.2013.10.108. [DOI] [PubMed] [Google Scholar]
- Schieber A.; Ullrich W.; Carle R. Characterization of Polyphenols in Mango Puree Concentrate by HPLC with Diode Array and Mass Spectrometric Detection. Innovative Food Sci. Emerging Technol. 2000, 1, 161–166. 10.1016/S1466-8564(00)00015-1. [DOI] [Google Scholar]
- Udomrati S.; Gohtani S. Tapioca Maltodextrin Fatty Acid Ester as a Potential Stabilizer for Tween 80-Stabilized Oil-in-Water Emulsions. Food Hydrocolloids 2015, 44, 23–31. 10.1016/j.foodhyd.2014.08.015. [DOI] [Google Scholar]
- Carmona Gallego J. A.Reología de Dispersiones Acuosas de Goma Xantana de Prestaciones Avanzadas. Ph.D. Thesis, University of Sevilla, 2015. [Google Scholar]
- Tabassum N.; Khan M. A. Modified Atmosphere Packaging of Fresh-Cut Papaya Using Alginate Based Edible Coating: Quality Evaluation and Shelf Life Study. Sci. Hortic. 2020, 259, 108853 10.1016/j.scienta.2019.108853. [DOI] [Google Scholar]
- Zhong Y.; Cavender G.; Zhao Y. Investigation of Different Coating Application Methods on the Performance of Edible Coatings on Mozzarella Cheese. LWT--Food Sci. Technol. 2014, 56, 1–8. 10.1016/j.lwt.2013.11.006. [DOI] [Google Scholar]
- Bertolo M. R. V.; Martins V. C. A.; Horn M. M.; Brenelli L. B.; Plepis A. M. G. Rheological and Antioxidant Properties of Chitosan/Gelatin-Based Materials Functionalized by Pomegranate Peel Extract. Carbohydr. Polym. 2020, 228, 115386 10.1016/j.carbpol.2019.115386. [DOI] [PubMed] [Google Scholar]
- Karaki N.; Aljawish A.; Muniglia L.; Bouguet-Bonnet S.; Leclerc S.; Paris C.; Jasniewski J.; Humeau-Virot C. Functionalization of Pectin with Laccase-Mediated Oxidation Products of Ferulic Acid. Enzyme Microb. Technol. 2017, 104, 1–8. 10.1016/j.enzmictec.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Kasapis S.; Bannikova A.. Rheology and Food Microstructure; Woodhead Publishing, 2017; pp 7–46. [Google Scholar]
- BeMiller J. N.Carbohydrate Chemistry for Food Scientists; Elsevier, 2018. [Google Scholar]
- Díaz Ocampo R.; García Zapateiro L.; Franco Gómez J. M.; Vallejo Torres C. Caracterización Bromatológica, Fisicoquímica Microbiológica y Reológica de La Pulpa de Borojó (Borojoa patinoi Cuatrec). Cienc. Tecnol. 2012, 5, 17–24. 10.18779/cyt.v5i1.79. [DOI] [Google Scholar]
- Kumar N.; Mandal A. Thermodynamic and Physicochemical Properties Evaluation for Formation and Characterization of Oil-in-Water Nanoemulsions. J. Mol. Liq. 2018, 266, 147–159. 10.1016/j.molliq.2018.06.069. [DOI] [Google Scholar]
- Matos M.; Gutiérrez G.; Martínez-Rey L.; Iglesias O.; Pazos C. Encapsulation of Resveratrol Using Food-Grade Concentrated Double Emulsions: Emulsion Characterization and Rheological Behaviour. J. Food Eng. 2018, 226, 73–81. 10.1016/j.jfoodeng.2018.01.007. [DOI] [Google Scholar]
- Silva-Weiss A.; Bifani V.; Ihl M.; Sobral P. J. A.; Gómez-Guillén M. C. Polyphenol-Rich Extract from Murta Leaves on Rheological Properties of Film-Forming Solutions Based on Different Hydrocolloid Blends. J. Food Eng. 2014, 140, 28–38. 10.1016/j.jfoodeng.2014.04.010. [DOI] [Google Scholar]
- Zhang L.; Liu Z.; Sun Y.; Wang X.; Li L. Effect of α-Tocopherol Antioxidant on Rheological and Physicochemical Properties of Chitosan/Zein Edible Films. LWT 2020, 118, 108799 10.1016/j.lwt.2019.108799. [DOI] [Google Scholar]
- Tian H.; Xiang D.; Li C. Tea Polyphenols Encapsulated in W/O/W Emulsions with Xanthan Gum–Locust Bean Gum Mixture: Evaluation of Their Stability and Protection. Int. J. Biol. Macromol. 2021, 175, 40–48. 10.1016/j.ijbiomac.2021.01.161. [DOI] [PubMed] [Google Scholar]
- Campelo P. H.; Junqueira L. A.; de Resende J. V.; Zacarias R. D.; Fernandes R. V.d. B.; Botrel D. A.; Borges S. V. Stability of Lime Essential Oil Emulsion Prepared Using Biopolymers and Ultrasound Treatment. Int. J. Food Prop. 2017, 20, S564–S579. 10.1080/10942912.2017.1303707. [DOI] [Google Scholar]
- Tan J.Overview: Semisolid Foods. In Rheology of Semisolid Foods; Joyner H., Ed.; Springer: Cham, 2019; pp 31–62. [Google Scholar]
- Báez L. A.; Santos J.; Ramírez P.; Trujillo-Cayado L. A.; Muñoz J. Development of Emulgels Formulated with Sweet Fennel Oil and Rhamsan Gum, a Biological Macromolecule Produced by Sphingomonas. Int. J. Biol. Macromol. 2019, 129, 326–332. 10.1016/j.ijbiomac.2019.01.114. [DOI] [PubMed] [Google Scholar]
- Vuillemin M. E.; Michaux F.; Adam A. A.; Linder M.; Muniglia L.; Jasniewski J. Physicochemical Characterizations of Gum Arabic Modified with Oxidation Products of Ferulic Acid. Food Hydrocolloids 2020, 107, 105919 10.1016/j.foodhyd.2020.105919. [DOI] [Google Scholar]
- Aljawish A.; Chevalot I.; Piffaut B.; Rondeau-Mouro C.; Girardin M.; Jasniewski J.; Scher J.; Muniglia L. Functionalization of Chitosan by Laccase-Catalyzed Oxidation of Ferulic Acid and Ethyl Ferulate under Heterogeneous Reaction Conditions. Carbohydr. Polym. 2012, 87, 537–544. 10.1016/j.carbpol.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Williams P. A.; Phillips G. O.. Handbook of Hydrocolloids; CRC Press: Boca Raton, 2000. [Google Scholar]
- Rao A. M.Measurement of Flow and Viscoelastic Properties. In Rheology of Fluid, Semisolid, and Solid Foods; Food Engineering Series; Springer: Boston, MA, 2014; pp 63–159. [Google Scholar]
- Sanz T.; Salvador A.; Hernández M. J.. Chapter 11 - Creep–Recovery and Oscillatory Rheology of Flour-Based Systems. In Woodhead Publishing Series in Food Science, Technology and Nutrition; Ahmed J.; Ptaszek P.; Basu S., Eds.; Woodhead Publishing: 2017; pp 277–295. [Google Scholar]
- Geremias-Andrade I. M.; Souki N. P. D. B. G.; Moraes I. C. F.; Pinho S. C. Rheological and Mechanical Characterization of Curcumin-Loaded Emulsion-Filled Gels Produced with Whey Protein Isolate and Xanthan Gum. LWT--Food Sci. Technol. 2017, 86, 166–173. 10.1016/j.lwt.2017.07.063. [DOI] [Google Scholar]
- Augusto P. E. D.; Ibarz A.; Cristianini M. Effect of High Pressure Homogenization (HPH) on the Rheological Properties of Tomato Juice: Viscoelastic Properties and the Cox–Merz Rule. J. Food Eng. 2013, 114, 57–63. 10.1016/j.jfoodeng.2012.07.025. [DOI] [Google Scholar]
- Su D.; Zhu X.; Adhikari B.; Li D.; Wang L. Effect of High-Pressure Homogenization on the Rheology, Microstructure and Fractal Dimension of Citrus Fiber-Oil Dispersions. J. Food Eng. 2020, 277, 109899 10.1016/j.jfoodeng.2019.109899. [DOI] [Google Scholar]
- Xu J.; Inglett G. E.; Chen D.; Liu S. X. Viscoelastic Properties of Oat β-Glucan-Rich Aqueous Dispersions. Food Chem. 2013, 138, 186–191. 10.1016/j.foodchem.2012.10.049. [DOI] [PubMed] [Google Scholar]
- Ramos A. M.; Ibarz A. Comportamiento Viscoelástico de Pulpa de Membrillo En Función de La Concentración de Sólidos Solubles. Cienc. Tecnol. Aliment. 2006, 26, 214–219. 10.1590/S0101-20612006000100034. [DOI] [Google Scholar]
- Hesarinejad M. A.; Koocheki A.; Razavi S. M. A. Dynamic Rheological Properties of Lepidium Perfoliatum Seed Gum: Effect of Concentration, Temperature and Heating/Cooling Rate. Food Hydrocolloids 2014, 35, 583–589. 10.1016/j.foodhyd.2013.07.017. [DOI] [Google Scholar]
- Morales-Contreras B. E.; Rosas-Flores W.; Contreras-Esquivel J. C.; Wicker L.; Morales-Castro J. Pectin from Husk Tomato (Physalis Ixocarpa Brot.): Rheological Behavior at Different Extraction Conditions. Carbohydr. Polym. 2018, 179, 282–289. 10.1016/j.carbpol.2017.09.097. [DOI] [PubMed] [Google Scholar]
- Bhushette P. R.; Annapure U. S. Physicochemical, Functional and Rheological Investigation of Soymida Febrifuga Exudate Gum. Int. J. Biol. Macromol. 2018, 111, 1116–1123. 10.1016/j.ijbiomac.2018.01.117. [DOI] [PubMed] [Google Scholar]
- Quintana S. E.; Machacon D.; Marsiglia R. M.; Torregroza E.; Garcia-Zapateiro L. A. Steady and Shear Dynamic Rheological Properties of Squash (Cucurbita moschata) Pulp. Contemp. Eng. Sci. 2018, 11, 1013–1024. 10.12988/ces.2018.8386. [DOI] [Google Scholar]
- Machacon D.; Quintana S. E.; Garcia-Zapateiro L. A. Viscous Characterization of Spreadable Pigeon Pea (Cajanus cajan) Paste with Antioxidants. Contemp. Eng. Sci. 2018, 11, 807–814. 10.12988/ces.2018.8375. [DOI] [Google Scholar]
- Picout D. R.; Ross-Murphy S. B. Rheology of Biopolymer Solutions and Gels. Sci. World J. 2003, 3, 105–121. 10.1100/tsw.2003.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari S. M.; Beheshti P.; Assadpoor E. Rheological Behavior and Stability of D-Limonene Emulsions Made by a Novel Hydrocolloid (Angum Gum) Compared with Arabic Gum. J. Food Eng. 2012, 109, 1–8. 10.1016/j.jfoodeng.2011.10.016. [DOI] [Google Scholar]
- Gyawali R.; Ibrahim S. A. Effects of Hydrocolloids and Processing Conditions on Acid Whey Production with Reference to Greek Yogurt. Trends Food Sci. Technol. 2016, 56, 61–76. 10.1016/j.tifs.2016.07.013. [DOI] [Google Scholar]
- Sharma S.; Rao T. V. R. Xanthan Gum Based Edible Coating Enriched with Cinnamic Acid Prevents Browning and Extends the Shelf-Life of Fresh-Cut Pears. LWT--Food Sci. Technol. 2015, 62, 791–800. 10.1016/j.lwt.2014.11.050. [DOI] [Google Scholar]
- Cortez-Vega W. R.; Brose-Piotrowicz I. B.; Prentice C.; Borges C. D. Influence of Different Edible Coatings in Minimally Processed Pumpkin (Cucurbita moschata Duch). Int. Food Res. J. 2014, 21, 2017–2023. [Google Scholar]
- Zambrano-Zaragoza M. L.; Mercado-Silva E.; Del Real L A.; Gutiérrez-Cortez E.; Cornejo-Villegas M. A.; Quintanar-Guerrero D. The Effect of Nano-Coatings with α-Tocopherol and Xanthan Gum on Shelf-Life and Browning Index of Fresh-Cut “Red Delicious” Apples. Innovative Food Sci. Emerging Technol. 2014, 22, 188–196. 10.1016/j.ifset.2013.09.008. [DOI] [Google Scholar]
- García M. A.; Pinotti A.; Martino M. N.; Zaritzky N. E.. Characterization of Starch and Composite Edible Films and Coatings. In Edible Films and Coatings for Food Applications; Springer: New York, 2009; pp 169–209. [Google Scholar]
- Peressini D.; Bravin B.; Lapasin R.; Rizzotti C.; Sensidoni A. Starch-Methylcellulose Based Edible Films: Rheological Properties of Film-Forming Dispersions. J. Food Eng. 2003, 59, 25–32. 10.1016/S0260-8774(02)00426-0. [DOI] [Google Scholar]
- Vargas M.; Chiralt A.; Albors A.; González-Martínez C. Effect of Chitosan-Based Edible Coatings Applied by Vacuum Impregnation on Quality Preservation of Fresh-Cut Carrot. Postharvest Biol. Technol. 2009, 51, 263–271. 10.1016/j.postharvbio.2008.07.019. [DOI] [Google Scholar]
- Li C.; Xiang F.; Wu K.; Jiang F.; Ni X. Changes in Microstructure and Rheological Properties of Konjac Glucomannan/Zein Blend Film-Forming Solution during Drying. Carbohydr. Polym. 2020, 250, 116840 10.1016/j.carbpol.2020.116840. [DOI] [PubMed] [Google Scholar]
- Singleton V. L.; Orthofer R.; Lamuela-Raventós R. M. B. T.-M. inE.. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Oxidants and Antioxidants Part A; Academic Press, 1999; Vol. 299, pp 152–178. [Google Scholar]
- AOAC . Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemist: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Quintana-Martinez S.; Morales-Cano A.; García-Zapateiro L. Rheological Behaviour in the Interaction of Lecithin and Guar Gum for Oil-in-Water Emulsions. Czech J. Food Sci. 2018, 36, 73–80. 10.17221/315/2017-cjfs. [DOI] [Google Scholar]