Figure 1:
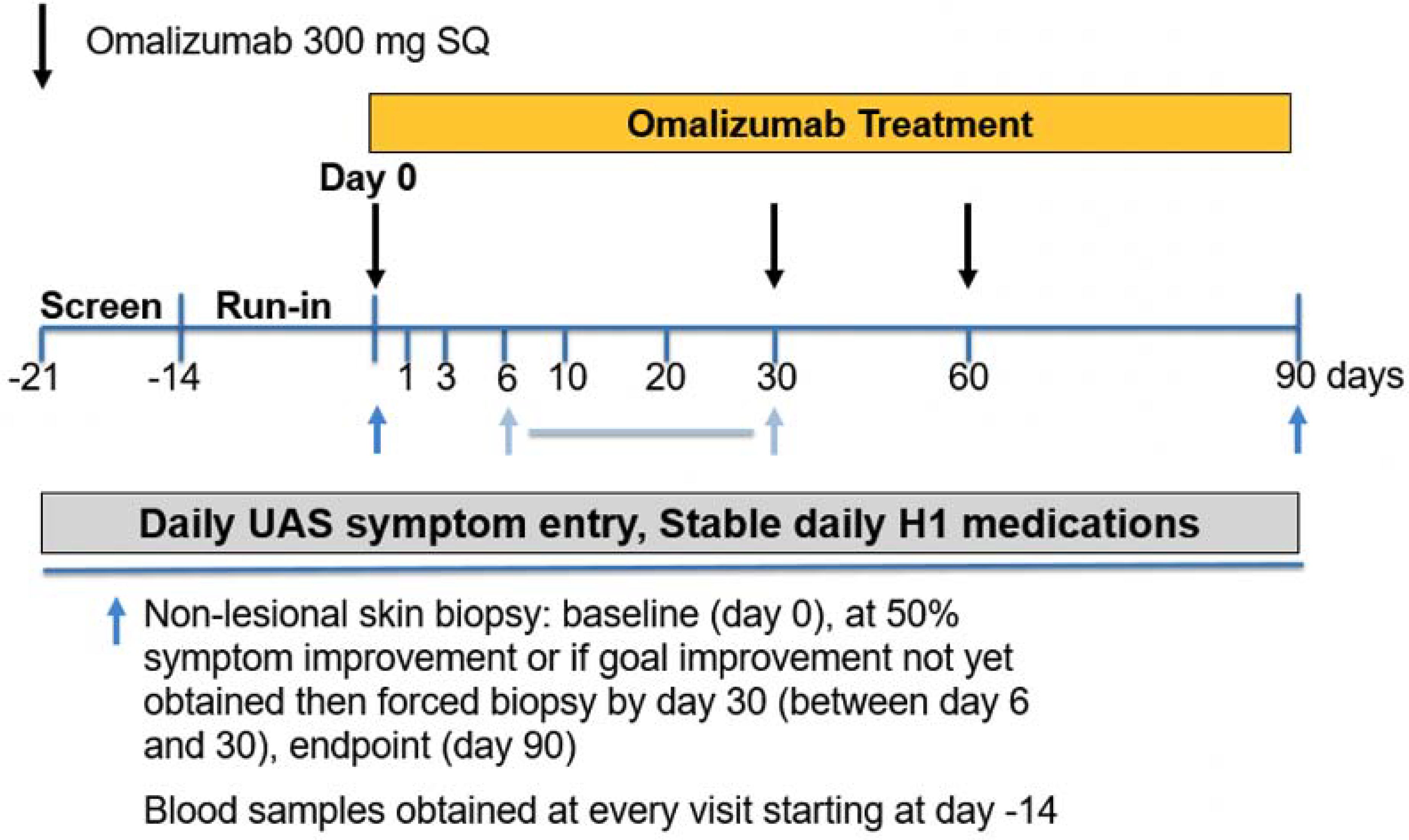
Study design. Depicted days indicate visits with laboratory studies. Black arrows indicate days of omalizumab dosing. Blue arrows indicate timing of skin biopsies. The second skin biopsy occurred between day 6 and 30 at the time of 50% symptom reduction relative to baseline UAS-7. If 50% symptom reduction was not achieved by day 30 then biopsy performed at day 30.
