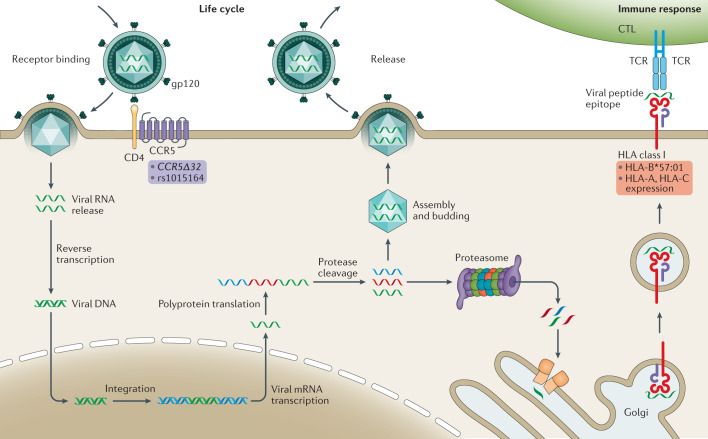
Fig. 1. Schematic representation of the HIV life cycle and the HLA-mediated host response.
The viral envelope glycoprotein gp120 binds CD4 and CC-chemokine receptor 5 (CCR5) on the surface of target cells triggering the fusion of the viral and host cell membranes; host genomic studies have implicated genetic variants in CCR5 (purple background) listed as modifiers of infectivity. Reverse transcription of the single-stranded RNA genome into double-stranded DNA (dsDNA) occurs using proteins carried by the infecting virion. Viral dsDNA is trafficked to the nucleus, where it is integrated into the human genome. The transcription of viral dsDNA results in viral gene expression and genome replication. Viral mRNA is translated into polyproteins, which are cleaved by the viral protease. Functional proteins assemble with copies of the viral genome at the cell membrane and mature virions bud from the surface. In parallel, as part of the immune response, viral proteins are digested by the host proteasome and processed through tapasin I and II (orange rectangles) into the Golgi, where the epitopes are loaded in the human leucocyte antigen (HLA) class I molecules. The peptide-loaded HLA protein is trafficked to the cell surface and presented to cytotoxic T lymphocytes (CTLs). Variability in epitope presentation by HLA-B alleles, such as the protective allele B*57:01, and in expression levels of HLA-C and HLA-A modify the response to infection and the set point viral load (red background). TCR, T cell receptor.