Figure 2.
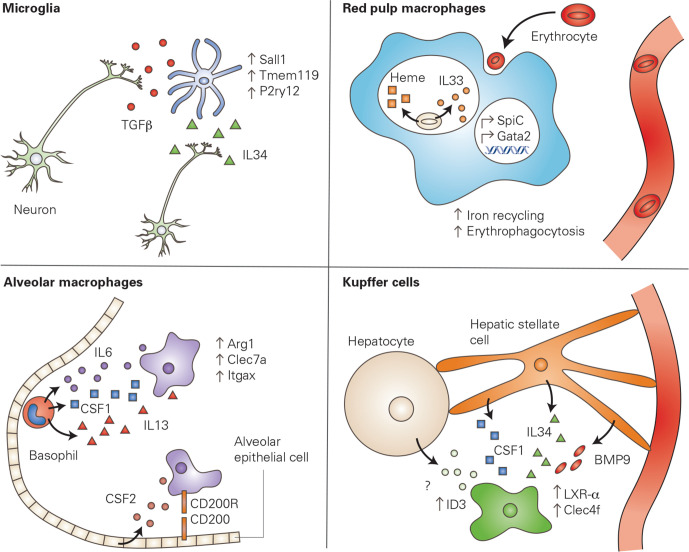
Local signals imprint macrophage phenotype. The local cellular environment produces signals which are able to activate specific transcriptional profiles within tissue resident macrophages, conferring a niche-specific phenotype or function to the macrophage. In the brain, neurons produce IL34, a growth factor required for the survival and proliferation of macrophages. In addition, neurons produce TGFβ, which has been shown to induce the expression of microglia-specific genes. In the spleen, red pulp macrophages clear aged erythrocytes via phagocytosis (erythrophagocytosis). The phagocytosed erythrocytes release mediators that upregulate transcription factors including SpiC and GATA2, leading to an increase of iron recycling and further erythrophagocytosis. Alveolar macrophages in the lung interact with alveolar epithelial cells via CD200-CD200R interaction. Epithelial cells are also a critical source of CSF2, a growth factor necessary for alveolar macrophage survival. Furthermore, basophils produce a variety of signalling molecules which are critical for the transcriptional signature of alveolar macrophages. Finally, Kupffer cells in the liver require multiple signalling molecules produced by hepatic stellate cells to upregulate Kupffer cell-specific genes, including Clec4f. Furthermore, hepatocytes seem to be necessary for the upregulation of Id3, another Kupffer cell-specific gene, however, the mechanism through which this signalling occurs is unknown. Kupffer cell panel adapted from Bonnardel et al 55 CSF1, colony stimulating factor 1; IL34, interleukin 34; TGFβ, transforming growth factor beta.