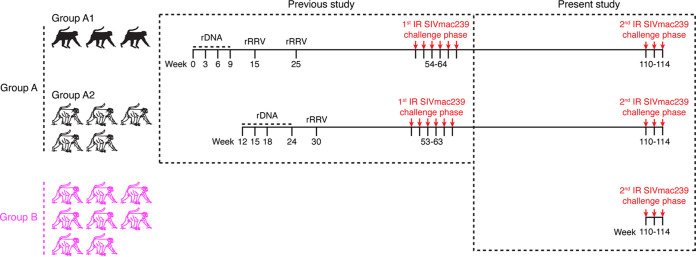
FIG 1.
Experimental design. The eight RMs in group A were used in a recent SIV vaccine trial conducted by our group (17). Those animals were part of a larger cohort of 16 RMs that were primed with rDNA plasmids (delivered by intramuscular electroporation) and then boosted with a mixture of five rRRV vectors expressing SIVnfl or SIV env. After six i.r. SIVmac239 challenges performed at weeks 53 to 64, eight of the 16 rDNA/rRRV-vaccinated RMs remained aviremic, whereas all (8/8) contemporaneous controls became infected. This difference in SIVmac239 acquisition between controls and vaccinees was statistically significant. To assess the durability of this vaccine-mediated protection, the eight rDNA/rRRV vaccinees that remained uninfected (referred to as group A here) were kept alive for another year without further boosters and then subjected to a second round of i.r. SIVmac239 challenges beginning at week 110. Eight additional unvaccinated RMs (group B) served as the concurrent controls for the second challenge phase, which utilized the same dose (200 TCID50) of the same stock of SIVmac239 used in the first challenge round. As part of our previous efforts to optimize the performance of the rDNA/rRRV vaccine regimen, three of the group A animals received two rRRV boosters (group A1), while the remaining five animals (group A2) were given one booster.