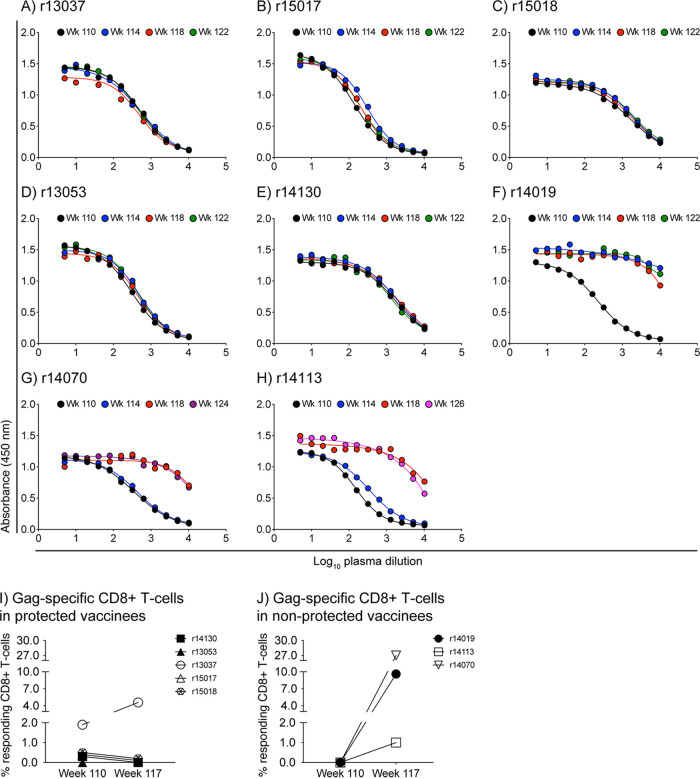
FIG 8.
Protected vaccinees show no evidence of anamnestic expansion of vaccine-induced SIV-specific immune responses after the second SIV challenge phase. (A to H) ELISA was used to determine the profile of gp120-binding Abs in serially diluted plasma from the group A animals. The samples used for this analysis were collected at week 110 (day of first SIV exposure), week 114 (day of third SIV exposure), week 118, and weeks 122 to 126. Data for the five protected vaccinees are shown in panels A (r13037), B (r15017), C (r15018), D (r13053), and E (r14130). Data for the three vaccinees that acquired SIV infection are shown in panels F (r14019), G (r14070), and H (r14113). (I and J) ICS was used to quantify Gag-specific CD8+ T-cell responses in PBMC from the group A animals at week 117, 3 weeks after the third SIV exposure. Those responses were then compared to those measured at week 110 in protected (I) and nonprotected (J) vaccinees. The percentages of responding CD8+ T cells shown on the y axes were calculated by adding the background-subtracted frequencies of positive responses producing any combination of IFN-γ, TNF-α, and CD107a.