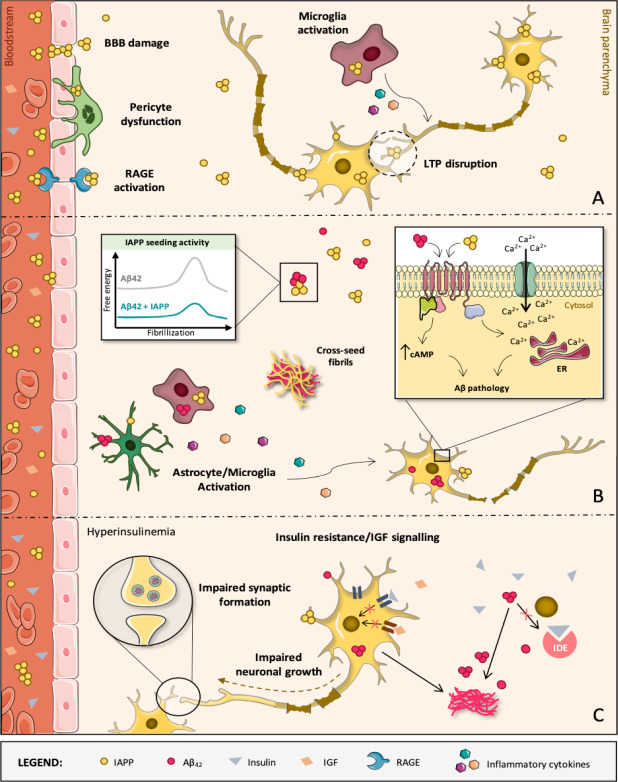
Figure 1.
Diabetes-induced molecular, cellular and structural alterations in the AD brain.
(A) IAPP oligomers are mostly formed within secretory vesicles of pancreatic β-cells, being then co-released with insulin into the blood stream in response to specific stimuli. IAPP oligomers are membrane-permeable and may compromise the BBB and diffuse into brain parenchyma. In brain microvascular pericytes of AD patients with T2D, IAPP forms intracellular toxic inclusions leading to nuclei fragmentation, autophagy impairment and loss of cell viability and function. IAPP oligomers may also engage receptor for advanced glycation end products, promoting inflammation and exacerbating cerebrovascular damage, namely to the BBB. It also facilitates toxic accumulation of IAPP in the brain, where IAPP can directly interact with neurons as well as microglia and astrocytes, activating them and contributing to AD pathology development. At high concentrations, IAPP can act upon neuronal receptors (e.g., AMY3) to modulate signaling cascades that are associated with long-term potentiation (LTP) disruption and, consequently, synaptic failure. (B) Despite IAPP being able to affect brain functions aside from Aβ42 pathology, IAPP and Aβ42 are clearly able to interact with each other, with IAPP accelerating Aβ42 aggregation and deposition. In fact, diabetic AD patients have cross-seeded fibrils and oligomers accumulated in the brain and constituted by these two peptides. Both individual and co-aggregates of IAPP and Aβ42 are prone to activate glial cells which, in response, produce and release inflammatory mediators (e.g., cytokines), creating a pathological environment detrimental for neurons. Direct activation of AMY3 neuronal receptors via IAPP and Aβ42 leads to increased cytosolic cAMP levels and downstream activation of molecular pathways (e.g., PKA, MAPK, AKT and cFos) involved in neuroinflammation, Aβ pathology and cell death. As a result, a Ca2+ perturbed influx may occur and disturb the ER homeostasis, contributing to neuronal apoptosis. (C) AD is also a metabolic disease in some instances. IDE degrades not only excess insulin in the brain but also other substrates, such as Aβ. If IDE is occupied with insulin, it is no longer free to degrade Aβ. This impairs Aβ clearance, causing senile plaques to be formed. When the brain loses capacity to deal with glucose, insulin and IGF, classical AD molecular biomarkers (Aβ aggregates) appear and negatively impact crucial neuronal functions. These events progressively impair brain homeostasis and promote massive neurodegeneration, leading to AD and dementia. AD: Alzheimer’s disease; AMY3: amylin-3 receptors; Aβ: amyloid beta peptide; BBB: blood-brain barrier; ER: endoplasmic reticulum; IAPP: islet amyloid polypeptide; IDE: insulin-degrading enzyme; IGF: insulin-like growth factor; RAGE: receptor for advanced glycation end products; T2D: type 2 diabetes.