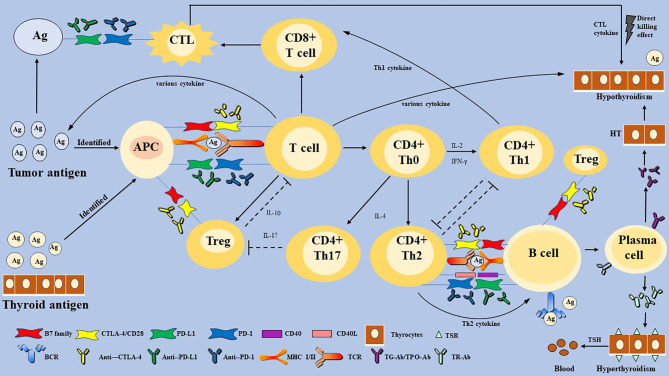
Figure 2.
The proposed mechanism of immune checkpoint inhibitors-related thyroid dysfunction. Thyroid IRAEs may involve T and B-lymphocytes, multiply cytokines, and diverse factors. Immune checkpoints are activated to escape the immune killing and clearance effect in most malignant tumor cells. Some immunotherapeutic agents can eliminate the inhibitory effect of T cells, which restore the anti-tumor response. However, activation of the immune system can also affect normal organ tissues, and lead to cell death, eventually leading to organ IRAEs. Thyroid IRAEs present mainly as hypothyroidism, hyperthyroidism, and transient thyroiditis, seem to overlap with AITDs. HT and GD are AITDs that cause hypothyroidism and hyperthyroidism, respectively. HT is caused by impaired immune tolerance of autoantigens, the destruction of thyroid cells. The pathogenesis of HT is considered to be a complex autoimmune process involving various activate and infiltrate T lymphocytes, B lymphocytes, and various cytokines. Then a cellular immune response and humoral immune response are induced, leading to direct thyroid injury and further thyroid antigen exposure. The main pathogenesis of GD can be understood as the combination of TSH receptor and TR-Ab secreted and released by Th2 cell-dependent B cells. Immune checkpoints are proposed to play a role in inhibiting the autoimmune process by inhibiting various immune cells. Whether thyroid IRAEs have the same mechanism as AITDs, warrants further elucidation. PD-1, programmed cell death gene-1; PD-L1, programmed cell death gene-1 ligand; CTLA-4, cytotoxic T-lymphocyte-associated antigen-4; MHC, major histocompatibility complex; Th cells, helper T cells; CTL, cytotoxic T lymphocyte cell; Treg, T-regulatory cells; APCs, antigen-presenting cells; TSH, thyroid-stimulating hormone; TPO-Ab, thyroperoxidase antibodies; TG-Ab, thyroglobulin antibody; TRAb, TSH receptor antibodies; AITDs, autoimmune thyroid diseases; HT, Hashimoto’s thyroiditis; GD, Graves’ disease.