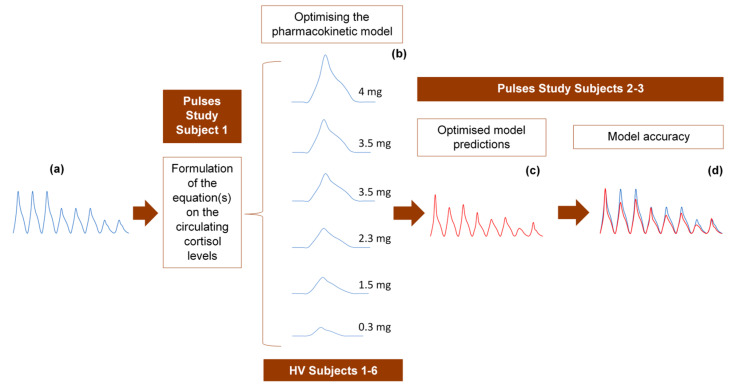
Figure 6.
Strategy for developing and optimising the pharmacokinetic model, describing the subcutaneous delivery of hydrocortisone (HC). (a) One of the 24-h cortisol profiles (blue diagram) was selected at random and used to formulate an equation describing plasma cortisol dynamics. We assumed constant rates of HC absorption and clearance and showed that such an approach cannot simulate reality. Three different scenarios were subsequently tested, of which only one was finally selected. (b) The selected optimised scenario assumes varying rates of HC absorption and clearance depending on the dose magnitude and circulating cortisol levels, respectively. The six 3-h healthy volunteer profiles (blue diagrams) under different subcutaneous doses of HC were used to validate these assumptions and confirm the reliability of the model. (c) Four plasma cortisol values from each of the remaining two patients with adrenocortical insufficiency were inputted into the model to predict their whole 24-h hormonal profile (red diagram). (d) The (theoretical) output of the model (red diagram) was compared to the actual 24-h plasma cortisol profile (blue diagram) of each of the two patients. HV: healthy volunteers.