Figure 4.
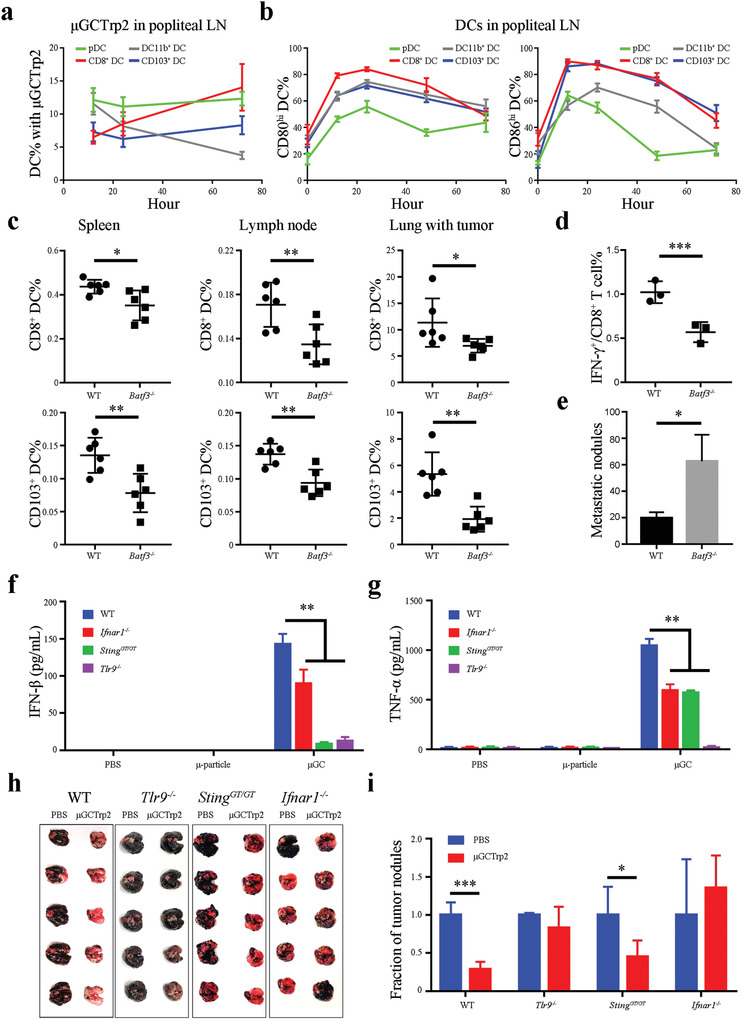
DC subpopulations and key genes in mediating vaccine particle transport and antitumor immunity. a) Time‐dependent transport of FITC‐labeled μGCTrp2 vaccine to popliteal lymph nodes by different subpopulations of DCs. Popliteal lymph nodes were collected from B16 tumor‐bearing mice 12, 24 or 72 h after they were treated with FITC‐labeled μGCTrp2 in the foot pads, and flow cytometry was applied to analyze percentages of FITC‐positive CD8+ DC, CD11b+ DC, CD103+ DC, and pDC. n = 3 mice per group. Error bars: mean +/− SD. b) Time‐dependent changes of maturation markers in DCs from popliteal lymph nodes. c) Flow cytometry analysis on levels of CD8+ DCs and CD103+ DCs in spleens, lymph nodes, and tumor‐bearing lungs from wild‐type (WT) and Batf3 −/− mice. n = 6 mice per group. Error bars: mean +/− SD. d) Flow cytometry analysis on levels of activated CD8+ T cells in popliteal lymph nodes from WT and Batf3 −/− mice treated with μGCTrp2 vaccine. n = 3 mice per group. Error bars: mean +/− SD. e) Quantitative analysis on metastatic tumor nodules in the lungs from WT and Batf3 −/− mice treated with μGCTrp2. n = 12 mice per group. Error bars: mean +/− SEM. f,g) Stimulation of cytokine expression in WT and mutant BMDCs. BMDCs from WT, Ifnar1 −/−, Sting GT/GT, and Tlr9 −/− mice were treated with μ‐particle or μGC for 24 h, and levels of f) IFN‐β and g) TNF‐α in growth media were measured and compared. n = 3 mice per group. Error bars: mean +/− SD. h,i) Antitumor activity in WT and mutant mice. WT and knockout mice bearing lung metastatic B16 tumors were treated twice with PBS control or μGCTrp2. Mice were euthanized on day 17, and lungs were collected and number of B16 tumor nodules was quantitated. Statistics: ANOVA for multi‐group comparison and Student's t‐test for comparison between two groups. *: p < 0.05; **: p < 0.01; ***: p < 0.001.
