Figure 4.
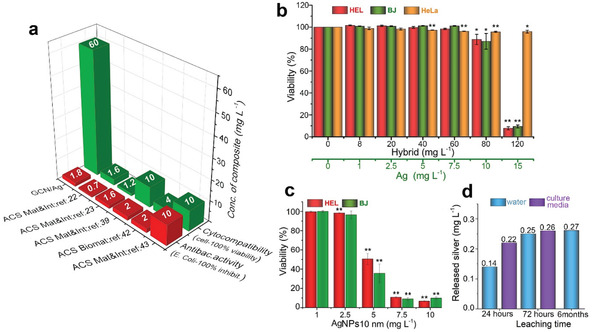
a) Comparative graph of the antibacterial activity and cytocompatibility of GCN/Ag in healthy human cells compared to representative examples from literature; in the latter case obtained on human cancer cell lines. Extended comparisons are also available in Table S1 (Supporting Information). b) Viability of human lung fibroblasts HEL, human skin fibroblasts BJ, and cancer HeLa cells treated with GCN/Ag, expressed in terms of hybrid (black line) and in terms of silver content (green line) (n = 3. c) Viability of HEL and BJ cells (n = 3) treated with 10 nm AgNPs. d) Leaching test of silver from GCN/Ag in water and in cell‐culture medium after 24, 72 h, and six months. The concentrations on the columns correspond to 0.07%, 0.11%, 0.13%, 0.13%, and 0.14% of Ag leached from the total amount of Ag (200 mg L−1 of Ag) that was initially contained in GCN/Ag which was added in the solution for the leaching test. *p ≤ 0.05; **p ≤ 0.01.
