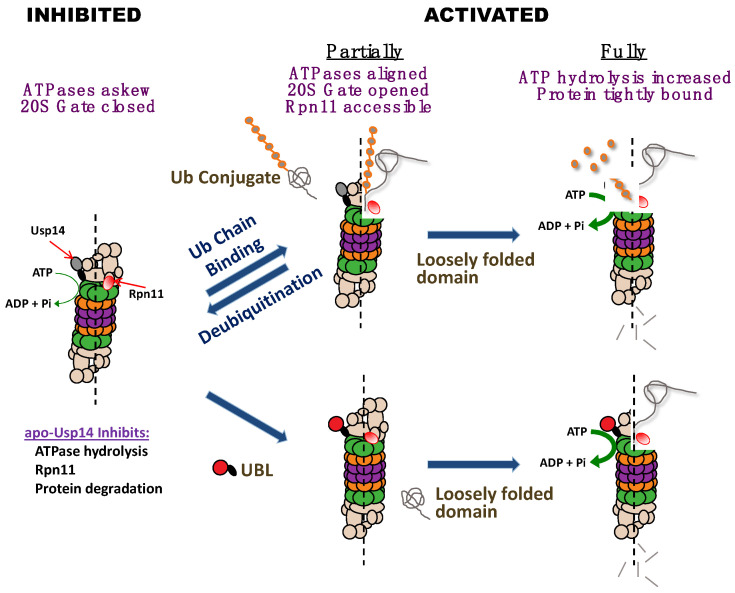
Figure 1.
26S proteasomes inhibited by Usp14 become activated either upon ubiquitin conjugate binding to Usp14 or upon binding another cell protein containing a UBL domain. In the absence of a ubiquitin conjugate, Usp14 inhibits several proteasome activities: peptide hydrolysis (due to the misalignment of ATPases and the closed 20S gate), ATP hydrolysis, Rpn11-mediated de-ubiquitination, and consequently protein degradation. However, upon binding to a ubiquitin conjugate, Usp14, through its UBL domain, activates proteasomes: the ATPases align, the 20S gate opens, and Rpn11 becomes accessible to substrates. If a loosely folded domain is also present in the substrate, proteasomes become fully active, ATP hydrolysis increases, and the protein substrate is bound more tightly, leading to processive degradation. Alternatively, the binding to the proteasome of a protein containing a UBL domain (e.g., a UBL-UBA shuttling factor) stimulates peptide hydrolysis. Full activation occurs if, in addition, there is present a protein with a loosely folded domain.