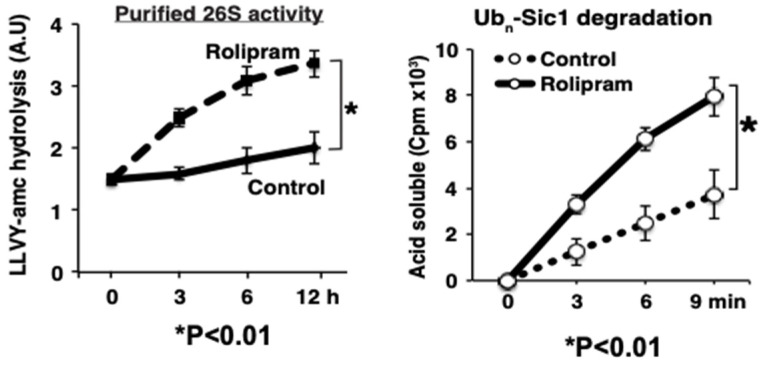
Figure 5.
26S proteasomes from mouse myotubes treated with the PDE4 inhibitor, rolipram, are more active in hydrolyzing peptides and a ubiquitinated protein. C2Cl2 myotubes were incubated with rolipram, and, at different times, samples were taken and 26S proteasomes purified by the UBL method (Left Panel). Chymotrypsin-like peptidase activity was increased in the treated cells. (Right Panel) After 6 h treatment of cells with rolipram, the purified proteasomes show a greater capacity to degrade ubiquitinated 32P-Sic1 (adapted from [15]). (Mean of three experiments ± SEM; * denotes p < 0.01).