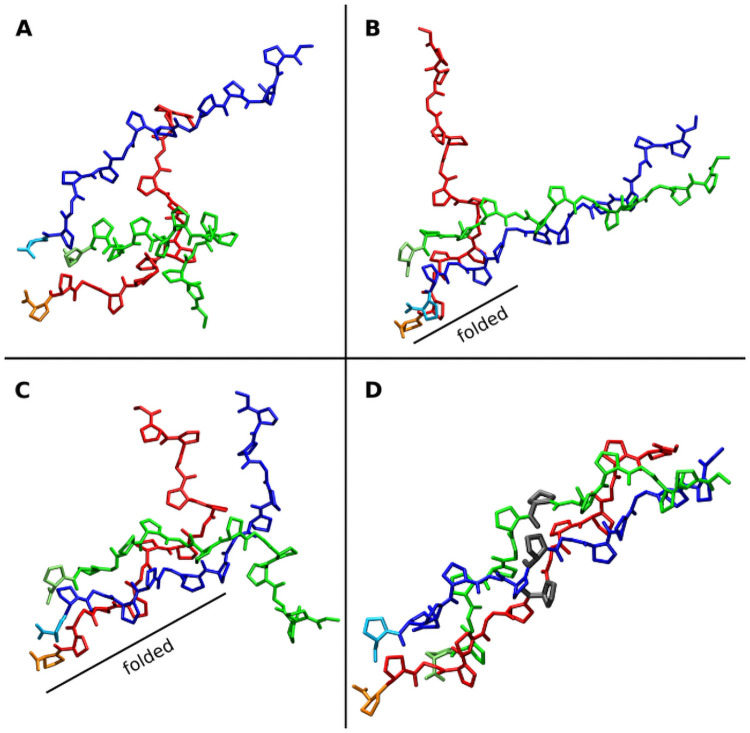
Fig 1. Representative snapshots from a collagen folding propagation simulation.
(A) Initial random arrangement of the three (Gly-Pro-Pro)5 chains (colored red, green and blue). (B) Folding nucleus formed at the C-terminus due to positional restraints on Cα atoms of the last two residues of each strand and partial formation of a near native structure of two chains (blue and green). (C) folding propagation with a near native arrangement of all three chains formed up to the middle of the complex. (D) Completely folded collagen triple helix (the 3 residues indicated in grey have the same residue number in each strand, are spatially close in the native structure and represent a residue triplet).