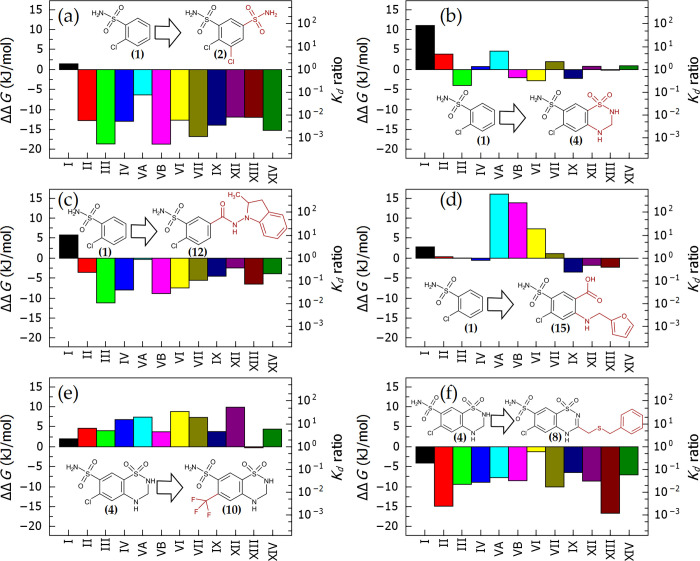
Fig 6. A comparison of two structurally related compound affinities.
The addition of chemical groups that are shown in red may enhance or diminish compound affinity for a particular CA isoform. This effect is shown on the left axis as the difference in the standard Gibbs energies of binding of both compounds corresponding to the ratio of the dissociation constants of both compounds on the right axis. The addition of some groups led to a nearly universal increase in binding affinity (a, f), or affected only a narrow subset of CA isoforms (d).