Scheme 1.
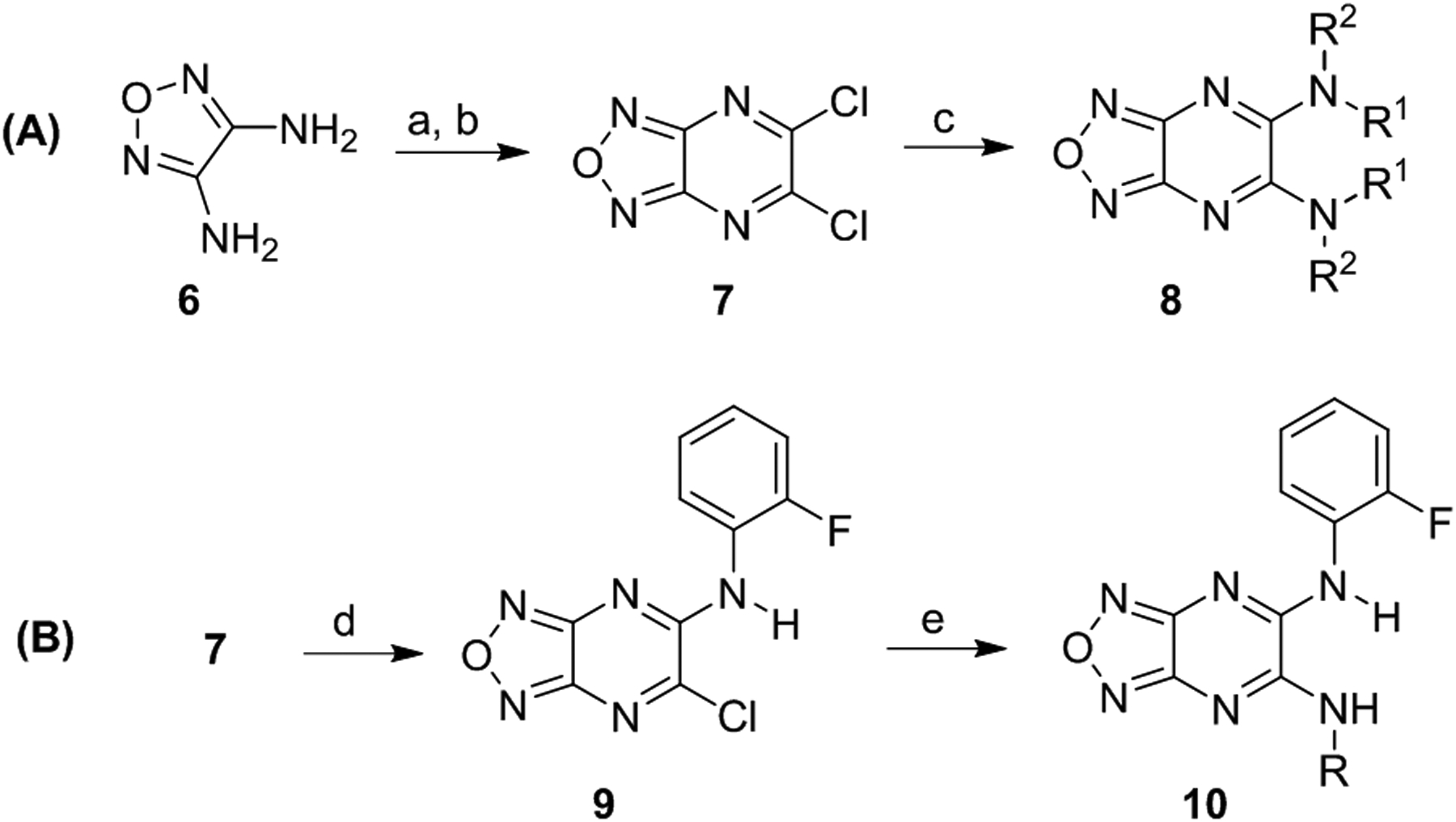
Synthesis of symmetrical (A) and unsymmetrical (B) derivatives. Reagents and conditions: (a) oxalic acid, 10% HCl, reflux, 3 h, quantitative; (b) PCl5, POCl3, reflux, 2 h, 30%; (c) aniline, THF, reflux, 19 h, 19 – 95%; (d) ortho-fluoroaniline, Et3N, THF, 0 °C, 0.25 to 1 h; (e) amine, Et3N, THF, 0 °C to rt, 19 h, 13 – 59%.
