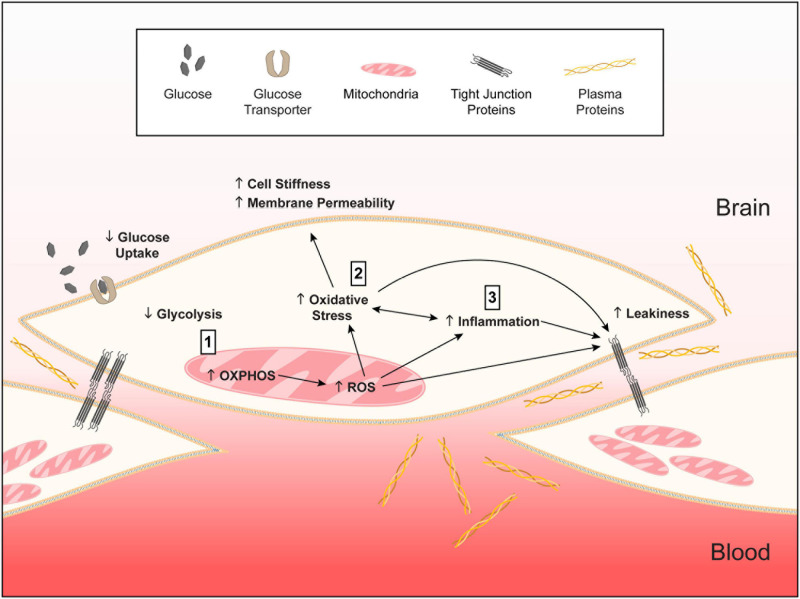
FIGURE 12.
Working model of APOE-modulated brain endothelial cell function. ↓ = lower and ↑ = higher for APOE4 compared to APOE3. We propose that (1) APOE4 brain endothelial cells have higher preference for oxidative phosphorylation (OXPHOS) compared to glycolysis. Lower glycolysis is evident in ATP rate assays, lower rate of glucose uptake and higher lactate production. Due to OXPHOS there is higher peroxisome and mitochondrial activity indicated by higher hydrogen peroxide degradation, mitochondrial membrane potential, citrate synthase activity and levels of electron transport chain complexes. Higher mitochondrial activity leads to the generation of reactive oxygen species, particularly superoxide, and lower levels of antioxidant systems, including heme and glutathione. (2) Higher reactive oxygen species lead to more oxidative stress to proteins (e.g., carbonylation) that must be cleared by proteasome activity. Oxidative stress to lipids (peroxidation, production of TBARS) alters membrane structures (lower phospholipids, higher ceramides) that manifests as higher cell stiffness and plasma membrane permeability. (3) Either in parallel or due to higher mitochondrial activity, inflammation pathways are activated with APOE4. APOE4-assocaited inflammation is characterized by chemokine production, immune cell adhesion and higher sensitivity of innate receptors to activation (e.g., TLR4). The combination of all these changes leads to higher basal transcellular permeability with APOE4. Therefore, autocrine signaling of apoE in brain endothelial cells represents a novel cellular mechanism for how APOE regulates neurovascular function. We further propose that this pathway is not necessarily detrimental in basal conditions for APOE4 and may even be beneficial for responding to infections and other stressors earlier in life. However, chronic changes in metabolic, mitochondrial, or inflammatory pathways in neurodegenerative conditions could lead to brain endothelial cell dysfunction.