Figure 2.
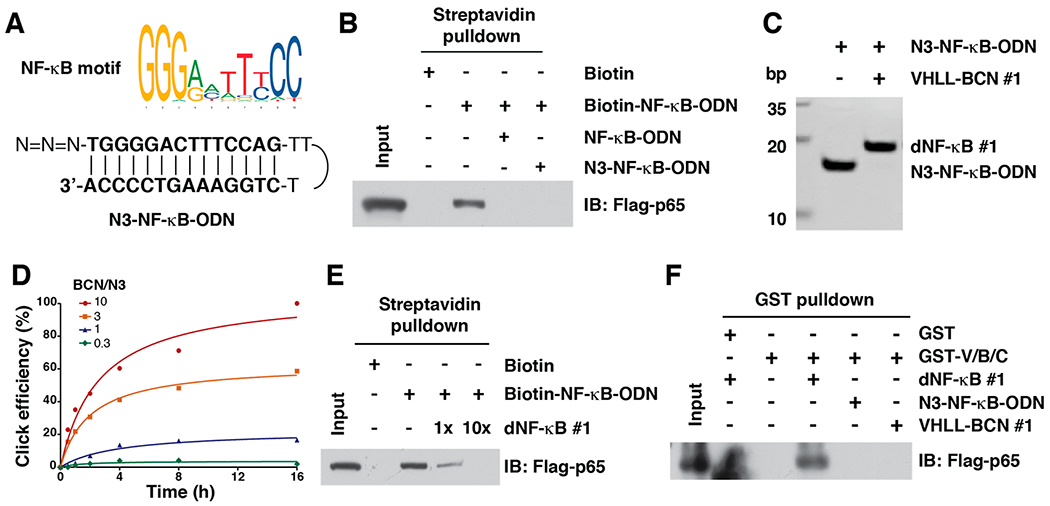
In vitro click reaction that leads to the generation of TF-PROTACs to target NF-κB. (A) A schematic diagram for the NF-κB motif and N3-NF-κB-ODN. (B) N3-NF-κB was capable of binding with NF-κB subunit p65. (C) Incorporation of VHLL-BCN #1 onto the N3-NF-κB-ODN led to an increase of molecular weight of 664 Da, which can be clearly separated by 20% native PAGE. (D) The click efficiency between VHLL-BCN #1 and N3-NF-κB-ODN with different ratios. A mixture of VHLL-BCN #1 and N3-NF-κB-ODN (50 μM) was incubated in PBS at 37 °C for indicated time points, followed by separation via 20% native PAGE. (E) dNF-κB #1 competed with Biotin-NF-κB for binding to RelA/p65. (F) dNF-κB #1 induced the VHL-TF-PROTAC-p65 ternary complex formation, which was determined by a GST pulldown assay.
