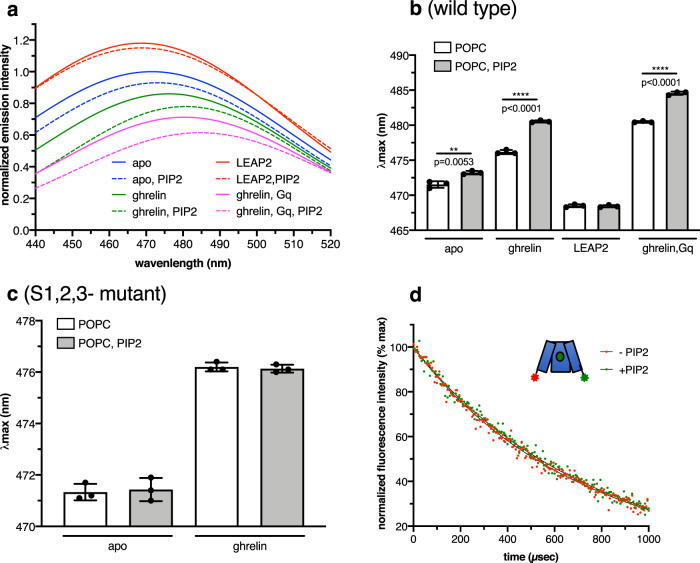
Fig. 3. PIP2 modulates GHSR conformation.
a MB emission spectra of GHSR in POPC or POPC:PIP2 (2.5% PIP2) nanodiscs in the absence of ligand, in the presence of 10 µM ghrelin, in the presence of 10 µM LEAP2(1-14) or in the presence of 10 µM ghrelin and the Gαqβ1γ2 trimer (1:5 receptor-to-G protein molar ratio). b, c Changes in λmax for the wild-type receptor and the mutants of the PIP2-binding sites. Data in (b) and (c) are mean ± SD of three experiments. Statistical values were obtained by means of unpaired Student’s t test **0.001 < p < 0.01, ***0.0001 < p < 0.001). d Intramolecular sensitized-emission decays from isolated ghrelin-loaded GHSR (10 µM ligand) in the absence of G protein and in the absence or the presence of PIP2 (2.5% molar ratio), with the donor and acceptor fluorophores in the cytoplasmic end of TM6 and TM1. Data are presented as normalized fluorescence intensity as a function of time and represent the average of three measurements. Source data are provided as a Source data file.