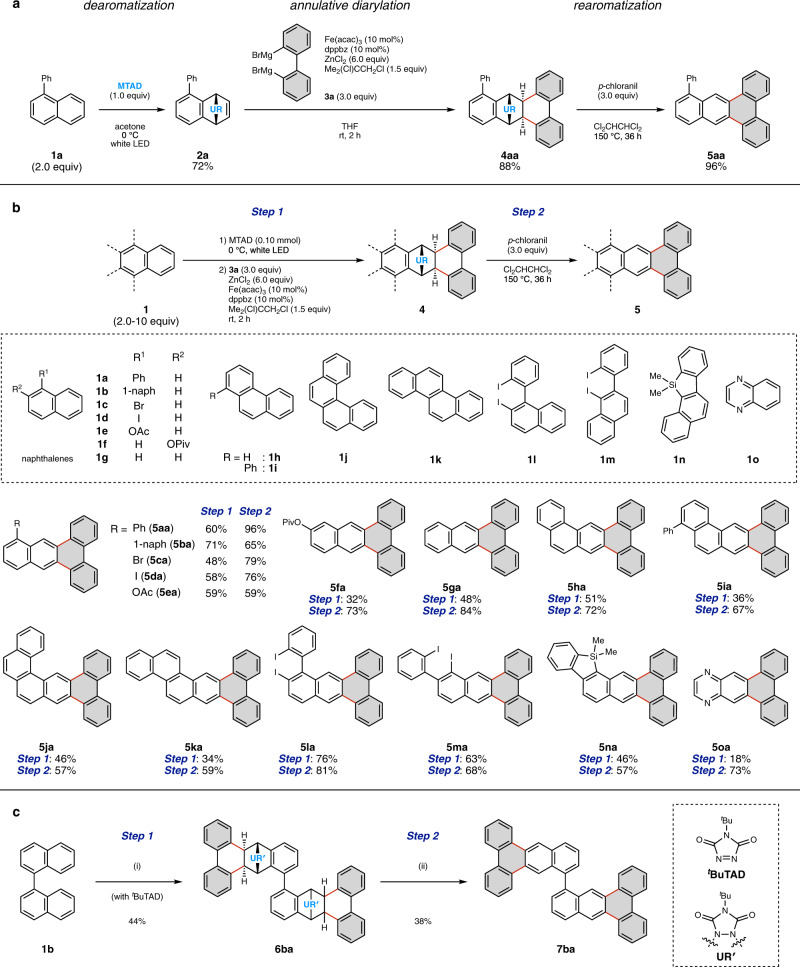
Fig. 2. M-APEX reactions enabled by dearomative strategy.
a Optimized reaction conditions. b Scope of aromatic templates. c Double M-APEX reaction of 1,1′-binaphthyl (1b). Reaction conditions. (i) 1b (1.0 equiv), tBuTAD (2.2 equiv), AcOMe, 0 °C, white LED, then 3a (6.0 equiv), Fe(acac)3 (20 mol%), dppbz (20 mol%), ZnCl2 (12 equiv), 1,2-dichloroisobutane (3.0 equiv), THF, rt, 2 h; (ii) 6ba (1.0 equiv), p-chloranil (6.0 equiv), 1,1,2,2-tetrachloroethane, 150 °C, 36 h. acac, acetylacetate; THF, tetrahydrofuran; Ac, acetyl. Gray hexagons represent newly constructed hexagons created by the present M-APEX reaction.