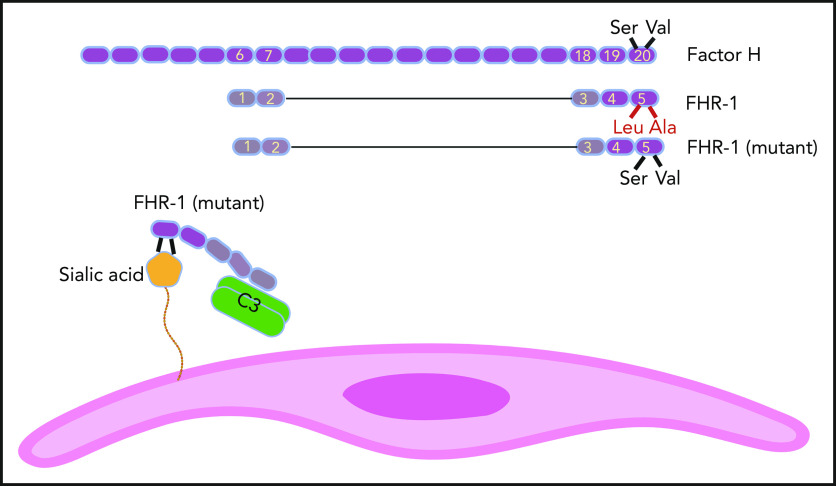
FH has 20 SCR domains, and FHR-1 has 5 of them. SCR domains of FHR-1 and FH are homologs, but FHR-1 does not possess complement regulatory domains SCR1 to 4 of FH. SCR 5 of FHR-1 is almost identical to SCR 20 of FH, except for 2 amino acids (Leu290 and Ala296 in FHR-1 instead of Ser1191 and Val1197 in FH). In some aHUS patients, these amino acids in FHR-1 are mutated back to their FH counterparts. As a result, mutant FHR-1 can bind with a higher affinity to sialic acid residues on the cell surface (eg, endothelial cells). Mutant FHR-1 molecules also bind C3 and bring it close to the cell surface, which promotes complement activation.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.