Abstract
The novel SARS-CoV-2 is expanding internationally. While the current focus is on limiting its transmission from direct contact with infected patients and surfaces during the pandemic, the secondary transmission potential via sewage should not be underestimated, especially in low-income and developing countries with weak wastewater treatment technologies. Recent studies have indicated SARS-CoV-2 positivity also be detected in the feces of patients. Therefore, the risk of transmission and infection can be increased into sewage by the fecal-oral way, mainly in some parts of the globe with a high amount of open defecation. This review collected scattered data and recent studies about the direct and indirect effects of coronavirus in the water cycle. The direct impacts of COVID-19 on wastewater are related to the presence of the coronavirus and suitable viral removal methods in different phases of treatment in wastewater treatment plants. The indirect effects of COVID-19 on wastewater are related to the overuse of cleaning and disinfecting products to protect against viral infection and the overuse of certain drugs to protect against virus or novel mental problems and panic to COVID-19 and consequently their presence in wastewater. This unexpected situation leads to changes in the quality of wastewater and brings adverse and harmful effects for the human, aquatic organisms, and the environment. Therefore, applying effective wastewater treatment technologies with low toxic by-products in wastewater treatment plants will be helpful to prevent the increasing occurrence of these extra contaminants in the environment.
Keywords: COVID-19, Coronavirus, Wastewater treatment, Cleaning products, Disinfectants, Pharmaceuticals
Graphical abstract
1. Introduction
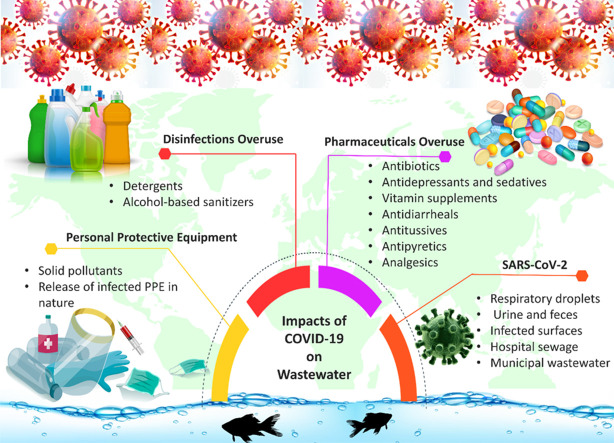
The crisis of COVID-19, which is caused by the new coronavirus SARS-CoV-2, is presently posing a considerable threat to the economic growth and the health of the world, especially developing countries. SARS-CoV-2 is a single-stranded virus with a positive-sense of RNA and spherical shape, which belongs to the Coronaviridae family [[1], [2], [3], [4], [5], [6]]. SARS-CoV-2 is more transmitted between the people rather than SARS-CoV and MERS-CoV, especially in dense population places such as public transportations, industrial processing factories, prisons, nursing homes, and etc. [[7], [8], [9]]. Despite the worldwide lockdown attempts, the rates of positive COVID-19 cases are still high. The World Health Organization (WHO) has reported the principal ways of coronavirus exposure are inhalation of respiratory droplets produced when a patient sneezes, coughs, and exhales or direct contact with infected surfaces [10]. Recent scientific researches announced the possible waterborne transmission of the SARS-CoV-2. Moreover, the presence of coronavirus RNA was confirmed by several reports in wastewater treatment plants (WWTPs) [11,12]. The risk of transmission by the fecal-oral way into sewage can also be a crucial concern and should be highlighted in the areas that have not adequate water treatment and sanitation facilities [12,13]. Even when the respiratory tract shows negative, stool may stay positive for SARS-CoV-2, and it raises a set of concerns about the possible presence of the virus in the effluent and choosing the appropriate methods for wastewater treatment to inactivate the virus [14].
Moreover, during the COVID-19 pandemic, the municipal wastewater can usually observe other excessive contaminants like disinfectants, pharmaceuticals, and disposable personal protective equipment, which are generally excreted by humankind activities [15]. The COVID-19 pandemic has also raised the consumption of certain drugs comprising antimicrobials and antidepressants between people due to improper attempts to protect themselves against the COVID-19 with antibiotics (while it is a viral sickness) and the worldwide occurrence of anxiety and depression. As a result, they can be found in the environment, particularly in the urban water cycle, which is harmful to aquatic organisms and the environment [[16], [17], [18], [19]].
The purpose of this review is to collect the present knowledge about the case of disturbances caused by COVID-19 in wastewater, both directly and indirectly, that can encourage researchers in various fields to conduct further research about this emerging problem. The direct existence of the COVID-19 in sewage and wastewater by human droplets, stool, or hospital wastes, the capability of transmission in this way, and effective methods to inactive the virus in the wastewater. Indirect effects of COVID-19 on wastewater include increased use of disinfectants, hand sanitizers, and surfactants (including detergents and soaps) and the over or misuse of certain medications (such as antimicrobials, antidepressants, etc.). Ultimately, collecting the efficient removal methods for these emerging wastewater contaminants is the other aim of this research.
2. Impact of COVID-19 on wastewater
2.1. SARS-CoV-2
Most viruses found out in stools, such as SARS coronavirus, can come from the superior respiratory tracts by deglutition respiratory secretions. The virus can be destructed by stomach acidity, but it may be protected when mixed with meals or potential resistor to acidic pH. Therefore, it can pass through the intestine. Moreover, it can derive from the replication of the virus in intestinal cells [20,21]. In 2002 and 2003, during the two outbreaks of SARS-CoV, up to 73% of infected people during the disease development had gastrointestinal symptoms, and the existence RNA of SARS-CoV was illustrated in the fecal specimens, even after ten weeks from the onset of symptoms [22,23]. Researches during the MERS-CoV pandemic showed that one-fourth of infected people announced symptoms, like abdominal pain or diarrhea, before severe respiratory symptoms, and in 14.6% of stool specimens, MERS-CoV RNA was detected [24,25]. Zhou et al. reported that intestinal mucous cells were extremely sensitive to MERS-CoV and could help viral replication [26].
Recent studies demonstrated SARS-CoV-2 positivity also be detected in the feces of patients in the absence of diarrhea and other gastrointestinal symptoms. For example, live SARS-CoV-2 was observed inside the stool of two infected persons without diarrhea symptoms [14]. Lescure et al. have also confirmed SARS-CoV-2 in urine specimens of patients [27]. Tang, A. et al. found SARS-CoV-2 from stool specimen of an asymptomatic ten-year child, which was evident for considering stool to be an extra routine diagnostic specimen separate from the respiratory tract samples [28]. The coronavirus viral concentration in feces of infected people who tested positive were exhibited in the range of almost 104–108 copies/L. However, in sewage, feces dilution is caused to reduce the viral load between almost 102–106.5 copies/L [29]. Although the possible infection of coronavirus is not proved, the existence of the virus is confirmed in the stool of a human until 33 days when the infected person's test is negative for SARS-CoV-2 [12]. The risk of infection increase in some parts of the globe and with a high amount of open defecation. According to UNICEF, 892 million people around the world still use open defecation [30]. For example, statistical data on November 2019 demonstrated that in India, about 28.7% of rural people still do not have access to any type of toilet [31]. Hence, a considerable amount of viruses would be expected in sewage from the number of diagnosed infected individuals, which has been exhibited in studies from many countries affected by this crisis. Currently, real-time reverse-transcriptase polymerase chain reaction (rRT-PCR), nested RT-PCR and quantitative RT-PCR (RT-qPCR), are the methods which are applied for the tracing of CVID-19 RNA [29] (Table 1 ).
Table 1.
Detection of SARS-CoV-2 in wastewater in some parts of the world.
| Location | Detection method | Type of sample | Positive sample | References |
|---|---|---|---|---|
| Milan and Rome, Italy | A novel nested PCR | Municipal wastewater | 6/12 | [34] |
| Louisiana, USA | RT-qPCR | Municipal wastewater | 2/7 | [35] |
| New York, USA | RT-qPCR | Municipal wastewater | 18/22 | [36] |
| Istanbul, Turkey | RT-qPCR | Municipal wastewater treatment sludges | 2/2 | [37] |
| 7/7 | ||||
| Municipal wastewater | 5/7 | [38] | ||
| Southeast Queensland, Australia | RT-qPCR | Municipal wastewater | 2/9 | [39] |
| Valencia, Spain | RT-qPCR | Untreated wastewater | 35/42 | [40] |
| Secondary treated water | 2/18 | |||
| Tehran, Iran | RT-qPCR | Treated wastewater samples of different WWTPs | 2/10 | [41] |
Additionally, medical wastewater of toxic and hazardous materials must be treated before sending out to the wastewater system. Sewage services in healthcare facilities must be managed precisely and in a different way after the COVID-19 crisis, especially in the areas with low financial and technical resources like Palestine and India or some regions of Africa [30,32,33]. Moreover, huge demand in consumption of personal protective equipment (PPE) such as masks, gloves, and shields, and improper disposal of this infected solid waste into the environment can increase not only the physical pollutants in water bodies but also can increase the risk of coronavirus transmission.
2.2. Cleaning and disinfecting products
Coronavirus can remain viable on surfaces such as stainless steel, cardboard, and plastic for hours or even days [42,43]; therefore, one of the best effective and practical ways to prevent and cope with the spread of coronavirus is to disinfect the surface and hands, and washing them successively. In recent research, it turns out that during the pandemic (from January to February 2020) in Wuhan city of China, almost 2000 tons of disinfectant were released into sewage systems [44,45]. These huge contaminated water, without any treatment, can directly be discharged into water bodies or can reduce the water quality and create significant problems for wastewater treatment. This situation not only brings a high danger risk for drinking water resources but also threatens the marine environment [46,47]. It turns out that excessive and successive use of disinfectants can increase drinking water consumption by 20%, which could lead to more than 15–18% wastewater generation [46]. Furthermore, the huge consumption of disinfectants, detergents, and soaps could find ways to access and contaminate water bodies which is one of the major environmental concerns related to the COVID-19 pandemic [46,47]. Antimicrobial resistance has become one of the other crucial concerns around the world due to the overuse of antimicrobials (antivirals, antibiotics, and antiparasitics) and extreme consumption of alcohol, surfactants, and hydrogen peroxides as the cleansing agents which are caused microorganisms resistance [48].
2.2.1. Surfactants
According to WHO, proper handwashing with soap and rubbing both sides of hands take at least 20–30 s [49]. The detriment to coronavirus spike proteins can lead to the inactivation virus [50]. Detergents and soaps are consist of surfactants, which are explained as the damaging factor for spike proteins or peplomers of the virus. For example, the Ebola virus, which indicates spike-protein mediated host attachment, can be inactivated by surfactant nanoemulsion; therefore, researchers have widely approved surfactants [51]. Surfactants are considered emerging pollutants that are not easily degradable and are stable in the environment for a long time. They are toxic for microorganisms and mammals in different environmental matrices [52,53].
In addition, during the pandemic, there are many other tasks, including disinfecting the indoor or outdoor environment, washing clothes, showers, and cleaning, which consume a large amount of water [46]. A single hand washes for each person might consume two liters of water if the tap is closed. This amount of water used can be increased to 4 L if the tap is remained open. Under these circumstances, each person consumes more quantity of water than before. For example, in Bundelkhand city of India, water demand was increased 60%–70%. In a similar way, massive operations of fumigation and disinfection have been done in all around words in order to disinfect avenues, streets, hospitals, markets. This situation contributed to huge wastewater generation, which mainly contains pollutants such as detergents [46].
The other main problem of common soap in hard water is sedimentation, which has led to the improvement of organic chemicals. In spite of the fact that the structural characteristics of these developed compounds are like soap, their chemical properties are different, and they can foam whenever they are being used in hard or acid water. Detergents formulation consists of several types of surfactants. Surfactants relied on their electrolytic dissociation and are mainly separated into four groups according to the charge on their head group, including non-ionic, amphoteric, anionic, and cationic [[54], [55], [56]]. Properties of surfactants can be defined by the head group of them [57]. Various used types of surfactants are shown in Table 2 , and several impacts of detergents on wastewaters are explained in Table 3
Table 2.
The classification of most commonly used surfactants and their applications [58,59,[60], [61], [62]].
| Type | Compound name | Abbreviation | Formula | Application |
|---|---|---|---|---|
| Anionic surfactants | Sodium dodecyl sulphate | SDS | NaC12H25SO4 | Cleaning and hygiene products |
| Sodium dodecyl benzenesulfonate | SDBS | C18H30NaO3S | As a detergent in cleaning products | |
| Linear alkylbenzene sulfonate | LAS | – | Household detergents | |
| Sodium alkyl sulphate | SAS | CnH2n+1OSO2ONa | Personal care products | |
| Alkyl ethoxy sulphate | AES | – | Personal care products | |
| sodium lauryl sulphate | SLS | C12H25NaO4S | Household detergents | |
| Alpha olefin sulfonate | AOS | CnH2n-1SO3Na | Excellent foaming and detergency characteristics/shampoos and other bath products | |
| Cationic surfactants | Quaternary ammonium compound | QAC | – | Found in disinfectant wipes, sprays, and other household cleaners |
| Benzalkonium chloride | BAC | – | Antiseptic and disinfectant | |
| Cetylpyridinium bromide | CPB | C21H38BrN | Antiseptic | |
| Cetrimonium bromide | CTAB | C19H42BrN | Topical antiseptic/antiseptic agent against bacteria and fungi | |
| Non-ionic surfactants | Alcohol ethoxylates | AE | – | Degreasers, emollients/detergents |
| Amphoteric surfactants | Cocamidopropyl betaine | CAPB | C19H38N2O3 | Personal care products |
Table 3.
Several impacts of detergents on wastewaters and the environment.
| Impact of detergents | Explanation | References |
|---|---|---|
| Reduction of dissolved oxygen levels | • Due to the incomplete degradation of surfactants, massive foam can be created in rivers and streams near dams. This layer of foam, on the surface of waters, reduces oxygen penetration rate from air into water, contributing to aquatic organisms becoming defective in the adsorption of dissolved oxygen. • Entering phosphate into water leads to eutrophication, causing the noticeable growth of algae, which is resulting in a decrease in dissolved oxygen levels. |
[63,64] |
| Detrimental effect on fish | • In high concentration, disrupt the fish vision • Damage to fish gills • Lead to alteration chemical and physical parameters of waters (pH, turbidity, salinity, and temperature), which reduce water quality and can affect the used dissolved oxygen concentration for fish. • Almost deadly for the fish at the detergent concentration above 200 ppm • Bringing signs of distress, slow swimming, and difficulty breathing in fish |
[63,65] |
| Detrimental effect on soil ecosystem | • Even though detergents are useful for photosynthetic function, their existence brings negative effects on the germination of the plants • Soil structure gradually destroyed • Plant health be affected by their negative effect • Increasing the pH of the soil, resulting in separation of soil elements • The electrical conductivity of soil be increased by irrigation with water containing detergent content |
[53,64,66] |
2.2.2. Hand sanitizer
Hand sanitizer can be separated into two main groups: alcohol-free or alcohol-based. Alcohol-based hand sanitizers are recommended by WHO during the COVID-19 pandemic due to their several advantages, including rapid action and protection against viruses and bacteria. This situation leads to the huge consumption of alcohol-based hand sanitizers. For example, in Japan, in April 2020, the production of alcohol disinfectants in the Kao company increased by 2000% to fight the shortage [67,45]. It turns out the overuse of hand sanitizer might become harmful to the environment and human health [68]. Overusing hand sanitizer contributes to antimicrobial resistance, putting more burdens on healthcare professionals, who already have been struggling with this problem [44]. The most effective formulation of hand sanitizer contains 62%–95% alcohol because it could inactivate viruses and denature the proteins of microbes. In general, alcohol-based hand sanitizers are mainly made up of isopropyl alcohols and ethanol [67,45]. Ethanol has a huge directly negative impact on aquatic organisms. Many kinds of research have been done to investigate the effects of ethanol on various species. In addition, the large amounts of isopropanol in water might lead to environmental impairment due to its high potency to decrease oxygen in the water, which ultimately brings adverse effects on the aquatic living system [69].
2.3. Pharmaceutical compounds
During the COVID-19 pandemic, the consumption of certain drugs, such as antibiotics, antidepressants and sedatives, drug of abuse, anabolic steroids, vitamin supplements, antidiarrheals, antitussives, antipyretics, and analgesics has increased among people. This increase in demand and use of the drugs has enhanced concern globally, which is affecting developing and even developed countries [16,[70], [71], [72]].
The consumption of antimicrobials in the prevention and therapy of COVID-19 is arising. A lot of people incorrectly try to protect themselves against the COVID-19 by self-medicate with antibiotics, despite it is a viral sickness. These attempts are mainly prevalent in developing societies [73,74]. The primary wave of COVID-19 widespread may have significantly affected antimicrobial utilization in hospitals. Silva et al. evaluated the antimicrobial consumption of specific hospital during the COVID-19 pandemic in Brazil. They found a 2500% higher rate of azithromycin consumption in the COVID-19 emergency department and a 2000% higher rate of azithromycin consumption in the clinical ward. Moreover, the usage of amoxicillin/clavulanate had a notably higher rate in all COVID-19 clusters [71]. The WHO and other specialists suggested that antimicrobial treatment should not be started for suspected or affirmed mild COVID-19 unless in critically COVID-19 patients with a high clinical suspicion of bacterial infection [75].
The increased antimicrobials usage can have critical outcomes with the existence of antimicrobial elements in the environment, which can stimulate resistance of the antimicrobials, and even in minimal amounts can present unpleasant impacts in non-target creatures. It has been indicated that above 80% of amoxicillin is discharged by urine from the person's body after 2 h, and consequently, the existence of amoxicillin in water sources and wastewaters can lead to harmful ecological problems [76]. A new typhoid fever epidemic in Pakistan has been related to the resistance of antibiotics due to inappropriate consumption of drugs, weak sanitation, and polluted water supplies [77].
Moreover, the novel COVID-19 pandemic has influenced people's mental health due to bereavement, fear lockdown, and loss of income in society, leading to an increase in depressive episodes in the countries such as UK, China, Spain, and the USA [16,78,79]. Antidepressants, which are considered in the classification of psychiatric drugs, are in the first step of therapy to treat depression, anxiety, and episodes which are resulting from the imposed quarantine. Psychiatric drugs are in the category of organic compounds which are comprising sertraline, carbamazepine, fluoxetine, citalopram, etc. [80]. Results indicated that European countries are the most antidepressants consumers in the world based on the Organization for Economic Co-operation and Development (OECD) data [81]. These drugs are relatively metabolized and excreted; hence, they enter into wastewater, and traditional treatments are not able to remove them. Varied literature confirms the existence of antidepressants in water sources worldwide and wastewater treatment plants, which can potentially be toxically bioaccumulated in the tissues of water organisms such as some aquatic plants, mollusks, and fishes [82]. The fate of these emerging pollutants, whenever released into the environment, depends on the physical and chemical nature of the pharmaceutical components. This issue is significant because several of these ingredients have been found in drinking water sources and wastewater effluents [70,83,84].
Nason et al. detected different chemicals and evaluated their trends from daily collected samples from March 19 to April 15, 2020, and weekly composite samples from March 19 to June 30, 2020, in primary wastewater sludge from WWTPs in New Haven, CT, USA. However, they did not know what chemicals were existed before analyzing samples. About antidepressant drugs, sertraline and citalopram had a clear increase in daily and weekly samples, respectively. Moreover, they found an increase in the concentration of some opioids and drugs of abuse such as methadone, hydromorphone, and cocaine in the weekly samples and increasing acetaminophen as a drug to relieve the symptoms of COVID-19 comprising headache and fever in their weekly sample analysis. The increased concentrations of hydroxychloroquine as a drug that received considerable attention due to its potential to treat COVID-19 were also obvious in daily sludge samples in the third week of investigation [72]. The specifications and environmental impacts of some drugs are collected in Table 4 .
Table 4.
Specifications and environmental impacts of some antidepressants, sedatives, and antimicrobials.
| Category type | Drug name | Drug bank ID | Structure | Impacts on water and the environment | Ref. |
|---|---|---|---|---|---|
| Antidepressants and sedatives | Sertraline | DB01104 |  |
• Endocrine-disrupting compound • Changes in crucial physiological functions such as behavior, growth, reproduction of aquatic species |
[72,85] |
| Citalopram | DB00215 | 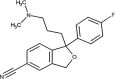 |
• Inducing significant foot detachment from the substrate in two kinds of freshwater snails | [86] | |
| Carbamazepine | DB00564 | 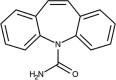 |
• Relatively limited acute toxicity on the studied organisms such as fish, algae, bacteria, and micro-crustaceans. | [87] | |
| Fluoxetine | DB00472 | 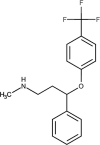 |
• Disrupt the endocrine systems of some aquatic creatures | [88] | |
| Diazepam | DB00829 |  |
• Detected in the tissues of some aquatic organisms • Disrupting behavioral traits of animals • Sublethal toxic effects in fish such as gene expression alterations |
[89] | |
| Antibiotics | Amoxicillin | DB01060 | 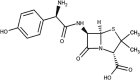 |
• Cause antibiotic resistance, decreasing the effectiveness of available antibiotics • Change microbial ecosystems potentially |
[76,90] |
| Azithromycin | DB00207 | 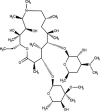 |
Therefore, the COVID-19 pandemic has huge negative direct and indirect impacts on water and wastewater quality. All of the already mentioned new contaminants can find a way to reach surface water and negatively affect the environment (Fig. 1 ).
Fig. 1.
The possible pathways of different contamination in water during the COVID-19 pandemic.
3. Wastewater treatment during COVID-19
The treated wastewater from treatment plants is usually discharged into receiving bodies of water or reused for purposes such as recreation and irrigation. This section deals with the introduction and evaluation of the effectiveness of different physical, biological, and chemical methods and unit operations that are currently used to remove organic contaminants and deactivate viruses. Hence, they can generally be used at the varied stages of the wastewater treatment during the COVID-19 pandemic.
3.1. Coronavirus removal
Preventing the spread of the virus in the environment all around the world is crucial before utilizing reclaimed water. There are three main phases to treat the infected water from coronavirus to safe water for recycling or reusing. The first treatment phase includes physical actions such as screening, grit chamber, and initial sedimentation in order to eliminate infected solids that are suspended in the wastewater. The second and third treatment phases consist of biological treatment actions to eliminate the biodegradable organic matter, and physicochemical treatment actions to further decrease turbidity, remaining organics, heavy metals, and pathogens such as coronavirus, respectively [91,92]. Although specific additional research about these methods on SARS-CoV-2, regular monitoring of their efficiency in real water treatment with considering all factors affecting virus survival and environmental considerations is needed to choose the best disinfection technology.
3.1.1. First treatment phase
The first treatment phase consists of physical processes that include the elimination of volatile and fixed solids suspended in sewage via physical barriers. Virus adsorption onto the large suspended solids in sewage, which is accompanied by gravitational sedimentation, is considered as the main and first mechanism in the treatment phase for the virus removal [93]. However, according to the available scientific reports, the gravitational sedimentation in the primary treatment phase is insufficient for the viral removal entirely from the sewage. The removal of coronavirus RNA through wastewater treatment operations has been seen for additional treatments comprising secondary and tertiary treatment, and tertiary treated wastewater is reused for irrigation and public domain directly [94].
3.1.2. Secondary treatment phase
Biological techniques such as membrane bioreactor, activated sludge, extended aeration biological processes, etc., are mainly used as part of the secondary treatment phase in the wastewater treatment plants [94]. Past studies have exhibited a higher elimination of the intestinal viruses by secondary treatment processes rather than the first treatment processes. Other studies have also reported the coronavirus resistance and survival in primary treatment was slightly higher than in secondary treatment due to the existence of higher organics in the primary treatment stage, which were protecting viruses from damaging [95,96].
In the activated sludge technique, uptake of viral particles on the organic biomass and elimination by sedimentation through the secondary clarifier assigned as the important and main mechanism for the virus elimination in the wastewater treatment [97].
The membrane bioreactor is used in the secondary treatment phase of wastewater to remove viral particles considerably, and it consists of membrane filtration and a suspended growth bioreactor. Membrane technology is cost-effective and environmentally friendly, with small or no chemical usage, a considerable decrease in the equipment size, and easy accessibility [98,99]. Based on recent studies, the high energy requirement is the major limitation of this technology, which is among 0.45 and 0.65 kWh·m−3 for the highest optimum performance [100,101]. Membrane bioreactor operations are designed with more retention times of solids towards activated sludge processes, which leads to varied treatment performances and other related conditions. The disadvantages of membrane compared to activated sludge are related to more operational difficulties and complexity [102]. Simmons et al. applied the membrane bioreactor process in the secondary treatment phase and could attain the log removal values of 6.8, 6.3, 4.8 for enterovirus, adenovirus, and norovirus, respectively [103].
SARS-CoV-2 is greatly similar to other coronaviruses, and they are seriously affected by decontaminants or various environmental parameters such as pH, temperature, presence of sunlight, and solids. According to current studies, coronaviruses are more fragile in the environment than intestinal viruses like norovirus, adenoviruses, rotavirus, and hepatitis virus, which exist in wastewater treatment plants [104,105]. The longer period time for retention in the wastewater treatment plants could assist the inactivation of coronavirus in swedge dramatically, and this effect is definitely expected to be further obvious at upper temperatures [106].
3.1.3. Tertiary treatment phase
The third phase of treatment includes operations such as coagulation, filtration, ultraviolet (UV), chlorination, ozonation, performic acid, nanomaterials, etc. [92]. Nanomaterials such as titanium dioxide, zero-valent iron, and carbon nanotubes (CNTs) have been applied for the inactivation and elimination of viruses in sewage [107,108]. The structure of the virus consists of a genome and, a protein capsid without or with an envelope. The initial purpose of the viral disinfection (such as UV, chlorination, ozonization, etc.) is to change one of these parts by applying environmental stress [105]. The viral envelope is more vulnerable to disruption. Hence, non-enveloping virus illustrates the upper resistance to inactivation and less susceptibility to unfavorable situations [109].
3.1.3.1. Chlorination
Free available chlorine was released through disinfection procedures, and its main sources are chlorine elements, chloramines, sodium hypochlorite, chlorine dioxide, calcium hypochlorite, and chloroisocyanurates. The presence of chlorine as hypochlorite ion (ClO-) and hypochlorous acid (HOCl) stay the most successful methods to combat viral particles. Hypochlorite is one of the powerful oxidizing agents which is oxidizing organic contaminants effectively, while undissociated hypochlorous acid is the microbiocidal agent primarily [110,111].
Wang et al. reported that the SARS-CoV was inactivated completely in sewage for 30 min at the free residual concentration of ClO2 equal to almost 2.19 mg/L or chlorine concentration greater than 0.4 mg/L. [95]. Chin et al. confirmed these results by some tests conducted in vitro on SARS-CoV-2, utilizing 1:99 diluted household bleach in order to inactive the virus after 5 min [42].
One of the major concerns and challenges in successful chlorination can be the existence of ammonia, which required chlorine for additional co-pollutants and pH. The Cl binds with the ammonia make a chlorine combination (chloramines), which is not effective and efficient enough against viral particles like free chlorine. Therefore, it is definitely pivotal to assure that Cl is not adsorbed by different demanding substrates such as organic matter, ferrous ion, ammonia, hydrogen sulfide, and nitrites. Organic matters normally neutralize chlorine-based materials, which cause short-term risks on the environment for plants and soil [112,113].
Randazzo et al. reported preliminary research on the existence of coronavirus RNA in wastewater after the second and third treatments. After normal activated sludge, about 11% of samples remained positive to coronavirus RNA; however, after the third treatment, 100% of samples changed to negative by using disinfection with NaClO, and in several cases, which can be combined with ultraviolet (UV) [40]. Zhang et al. used a high dose of sodium hypochlorite to disinfect hospital sewage from SARS-CoV-2; however, this method had a high level of residual by-products, which possessed notable environmental risks [114].
3.1.3.2. Performic acid
Performic acid is a less-stable common disinfectant and oxidizing agent with the formula CH2O3, which is a combination of hydrogen peroxide (35%) and formic acid (10 to 20%) [115]. It has many benefits over chlorine and can be more efficient to inactive viruses, bacteria, and fungus during wastewater treatment [116]. Performic acid also can apply at conditions with low-temperature (under 25 °C); hence, it can be used in cold areas or during winter [117]. Performic acid decomposes quickly, which leads to enhance the amount of reactive oxygen and consequently making more powerful disinfection and produce non-toxic H2O2 and formic acid as by-products [118].
3.1.3.3. Ultraviolet (UV)
UV radiation effectively controls the growth of microbial in any medium, like air, water, and any kind of surface. Currently, different environmental public settings around the world, such as health care facilities, hospitals, airports, and shopping centers, are considering the UV disinfection devices implementation for disinfection of the surfaces that are frequently touched and streams of circulating air. However, the applied UV-C energy dosage must be balanced in order to obtain acceptable biocidal efficiency levels and prevent excessive energy that can damage the surfaces and reduce the predicted lifetime [119].
In recent years the ultraviolet disinfection technique is more interested in water treatment because it is a clean disinfectant and effective against most waterborne pathogens comprising several microbial pollutants that are relatively resistant [120]. Moreover, this method can easily operate and install, and it is non-corrosive. The viral particles under the UV light lose their potential to infect and replicate by damaging the genome and protein comprising the break down of the phosphodiester bonds and the links with extra molecules [121]. Darnell et al. showed the partial inactivation of SARS-CoV by UV-C light irradiation (254 nm, dose 4016 μW cm−2), which could reach to 400-fold reduction in infectious viral load at 1 min with enhancing efficiency up to 6 min. The viral particles became totally inactivated after almost 15 min below the limit of the detection, while no impacts of UV-A (365 nm, with dose 2133 μW cm−2) exposure were observed on the virus over a 15 min period of the exposure [122]. Disinfection efficiency of UV may be reduced dramatically by algae with biofouling of the lamp, colored and turbid substances protecting microorganisms [123]. Hence, the secondary treatment followed by UV helps residual protection and assures microbial shielding redundancy.
3.1.3.4. Ozonization
Ozone is a clean and potent oxidizing factor, which is an effective microbicide against protozoan, bacteria, and viruses by attacking viruses and destroying the viral protein. The ozone technique has exhibited excellent performance against non-enveloped and enveloped viruses in water, aerosols, and surfaces, comprising analogous viruses in morphology to SARS-CoV-2 [124,125]. Ronaldo et al. assessed the virucidal performance of ozonated water, which is a strong oxidizing factor, against SARS-CoV-2. The source of ozonated water at a low concentration (0.2–0.8 ppm) was the particular faucet, which demonstrated the reduction of two log10 in virus infectiveness after exposure to ozonated water for about 1 min, in comparison to control groups [126].
The combination of treatment techniques comprising UV treatment, ozonation, and chlorination could inactive about almost 99.99% of fecal coliform in water, and no coronavirus was found after disinfection. The studies revealed that applying the ozonation technique enhanced transmittance of UV by almost 20–30% inside the water, and as a result, UV dose can be decreased [127].
3.2. Removal of other emerging contaminants
In general, conventional treatment systems that are existing today might not be able to completely remove these huge amounts of organic micropollutants during the COVID-19 pandemic in urban wastewaters. Although additional research is needed, various pharmaceuticals components were detected in the inlet and outlet waters in different wastewater treatment plants in the past, which means several kinds of these substances could not be removed effectively by existing systems. Hence, more specific and effective treatments are needed in order to reduce the environmental impacts of sewage. Proper water treatments include chemical precipitation to remove phosphorus, ionic exchange for removing ions, biological systems to eliminate nitrogen, AOPs for removing toxic organic compounds, distillation for volatile organic compounds removal, and adsorption for removal of inorganic and organic pollutants. Some of these water treatment methods have not to be applied on a large scale and are under research because there is a lack of information about their reactor design issues, proper data about their mechanisms, and the impacts of operational variables [46,128,129]. In the following, different water treatment methods with proper efficiency for cleaning and pharmaceutical compounds are discussed, and the advantage and disadvantages of each method are mentioned in Table 5 . Moreover, some of the related studies with these methods are summarized in Table 6 .
Table 5.
Advantages and disadvantages of the organic contaminants removal methods.
| Treatment | Advantage | Disadvantage | References |
|---|---|---|---|
| Adsorption | • Easy to operate • Highly efficient • Cost-effective • Environmentally friendly |
• Surfactants can be only separated from the wastewater but cannot be destroyed • Need skilled labor |
[174,175] |
| Advanced Oxidation Processes (AOPs) | • Rapid reaction rates • Small footprint • Mineralization of organics |
• Removal of residual peroxide may need to be considered | [176,177] |
| Biological treatments | • Low cost • Easy application • Can be improved by combining with membranes (membrane bioreactors) |
• Sludge generation during the treatment • High retention time |
[178,179] |
| Coagulation and flocculation | • Easy application • High pollutant removal efficiency |
• Transferring toxic compounds to the solid phase | [180] |
Table 6.
Some of the related studies for mentioned wastewater treatment methods.
| Types of treatment | Pollutant name | Description | Adsorption capacity/removal capacity | References |
|---|---|---|---|---|
| Adsorption | CTAB | Adsorbent: polymer resin Lewatit VPOC | 250 mg/g | [181] |
| CTAB | Adsorbent: natural zeolite (clinoptilolite) | 284 mg/g | [182] | |
| CTAB | Adsorbent: activated carbon (Merck) | 207 mg/g | [183] | |
| SLS | Adsorbent: amino cross-linked chitosan microspheres (ACCMs) | 888 mg/g | [184] | |
| SDS | 825 mg/g | |||
| SDS | Adsorbent: chitosan hydrogel | 76.9 mg/g | [185] | |
| Carbamazepine | Adsorbent: BGO-CS | 11.2 mg/g | [186] | |
| Carbamazepine | Adsorbent: MOF | 99% | [187] | |
| Acetaminophen | Adsorbent: BGO-CS | 13.7 mg/g | [186] | |
| Amoxicillin | Adsorbent: ACAF | 90% | [188] | |
| Advanced Oxidation Processes (AOPs) | SDS | Method: UV-H2O2 | 100% | [156] |
| Amoxicillin | Method: UV/H2O2/TiO2 | 70.9% | [189] | |
| Carbamazepine | Method: PS/Fe(II)/UV–vis | 100% | [190] | |
| Biological treatments | LAS | Activated sludge process | 99% | [161] |
| AE | Anaerobic sludge | 99% | [191] | |
| LAS | 71.10% ± 11.3 | |||
| Coagulation and flocculation | Effluent sample including various surfactants | – | 99% | [180] |
3.2.1. Adsorption
In order to separate the interface of two phases, adsorption can be used with two classifications: chemisorption and physisorption, depending on the adsorbate and adsorbent interactions. One of the significant advantages of the adsorption method is the regeneration or recycling of adsobents, which makes it economical for use in water treatment. The reusing of the adsorbent mainly relied on the regeneration efficiency of the adsorbent. The pharmaceuticals and surfactants adsorption depends on the properties of the solution, adsorbent, and adsorbate. For example, surface charge, pore volume, pore size, and surface area of the adsorbent, and continuously the polarity, size, molecular weight, hydrophobicity, functionality, geometry, and solubility for adsorbate in an aqueous solution [[130], [131], [132], [133]]. Many different adsorbents have been synthesized for the removal of pharmaceutical compounds and surfactants from water.
The nanomaterials can perform very well for surfactant adsorption. Ncibi et al. used carbon nanotubes (CNTs) with adsorption capacities of 156 and 312 mg/g for removal of CTAB and SDBS surfactants. The performance for the removal efficiency of surfactants can be increased by ultrasonication. [134]. Also, carbon nanotubes are effective adsorbents for removing pharmaceutical compounds [135]. However, carbon nanotubes in the largescale application are limited because of several problems such as difficult separation contribute to leakage into water bodies and eventually has adverse effects on human health and ecosystems [134].
Activated carbon can be considered as one of the most effective materials for the adsorption of surfactants and pharmaceutical compounds from wastewater [[136], [137], [138]]. Schouten et al. reported that activated carbon which has a proper microporous structure in the average pore diameter of almost 1.5 nm, can remove anionic surfactants such as LAS and AOS. The pore sizes and ionic strength are important factors for this process. The macro (>50 nm) and meso (2–5 nm) pores are suitable for the adsorption of AOS and LAS. It turns out that activated carbon and double-layered hydroxide (LDH) can be considered as the proper candidate for the adsorption of the anionic surfactant due to several properties such as being cost-effective and having high adsorption capacity. The ionic interactions caused higher adsorption in comparison with non-ionic interactions [139]. The effect of pore and particle size on the anionic surfactant adsorption by four activated carbons which have various pore sizes, indicated that the activated carbon with a smaller pore size between 0.56 and 0.77 nm could adsorb more anionic surfactant in comparison with other activated carbons. The particle size has a remarkable effect on the small particle due to the complex compound formation between cations of surfactant molecules and activated carbon. Although using activated carbon has several drawbacks such as inefficient regeneration, poor adsorption selectivity, and expensive synthesis. However, taking advantage of economical raw materials which have efficient regeneration ability for the synthesis of activated carbons can improve this adsorbent for the removal of surfactants [140,139]. Moreover, according to the literature, activated carbons have a high performance in the adsorption of pharmaceuticals. One of the advantages of activated carbon for removing pharmaceuticals is that pharmacologically or toxic active products do not generate [129].
Chitosan is a biocompatible, biodegradable, and non-toxic mucoadhesive biopolymer. It is an economical, renewable, and generally available biomaterial and, after cellulose, is the most abundant natural polysaccharide [141]. Parhizgar et al. investigated that this adsorbent could successfully remove 97% of the SDBS (anionic) surfactant, and its adsorption capacity was almost 6.38 mg/g [142]. Generally, from literature results, the unmodified chitosan has low adsorption capacity in comparison to modified chitosan for anionic surfactants. Chitosan can be modified with various methods such as doping with metals or cross-linking contribution to the higher surface area to having more active sites [143].
3.2.2. Advanced oxidation processes (AOPs)
AOPs can degrade almost all kinds of organic pollutants into harmless products. This method is considered an environmentally friendly process because pollutants could not transfer from one phase to another one, and massive, hazardous sludge does not be produced in this method [144,145]. AOPs can reduce organic pollutant concentration from hundreds ppm to less than near 5 ppb [146]. This method relies on the generation of OH• (reactive hydroxyl radicals). After series of oxidation reactions, ultimate mineralization products, which are CO2 and H2O, are produced. AOP can mix with ozone (O3), Fenton's reagent (H2O2/Fe2+), photocatalysis, and hydrogen peroxide (H2O2) and combine with ultraviolet (UV) irradiation, like TiO2/UV, ozone/H2O2/UV, ozone/UV, and H2O2/UV. Methods that include UV radiation can be considered as photochemical processes. UV source improves the reduction of Fe2+ and Fe3+, which can be reacted with H2O2 in order to produce more hydroxyl radicals [147,[148], [149], [150]].
Some studies have been done with using AOPs as an effective treatment for pharmaceutical pollutants [130,151] and surfactants [152,153]. Mechanisms of degradation might vary from one method to another one. The Fenton-based AOPs popularity has been much dependent on the selection of iron-based catalysts for water treatment. Although, due to the limitation in the solubility of iron species, the efforts of research have focused on the development of iron-free Fenton systems for the H2O2 activation. However, one disadvantage of using Fenton's technique is sludge formation, which contains iron hydroxide as a secondary product. Thus, sludge disposal should be considered in the initial cost and design process. Moreover, the Fenton process requires continuous pH adjustment because this process needs a lower pH [[154], [155], [156]]. The AOPs are recommended to be applied in new or existing wastewater treatment plants. For highly concentrated wastewater, AOPs are suitable to be considered as pre-treatment to decompose recalcitrant organic contaminants into biodegradable intermediates or products that can later be eliminated in biological post-treatments [154].
3.2.2.1. Ultrasonic
Ultrasonic irradiation (sonochemistry) is a promising technique for the degradation of various types of hazardous organic compounds and pollutants from wastewater with undesirable impacts in the water environment. Ultrasonic irradiation can commence pyrolytic and oxidative degradation procedures. This method does not need any chemical additives and can successfully apply to sludge, colored or turbid solutions. Ultrasonic irradiation exposure induces acoustic cavitation in water. Sonochemistry is in the category of AOPs, a phenomenon that is related to the production, growth, and intense collapse of gaseous bubbles into water (known as cavitation) in the pressure (~1000 atm) and high temperature (~5000 K) conditions. Irradiation of water with ultrasound decomposes water molecules into highly reactive radicals, including •Ḥ and •OH. These reactive radicals can react with each other or diffuse to consider as an oxidant [[157], [158], [159]].
Kıdak and Doğan removed and degraded antibiotic amoxicillin by ultrasonic irradiation alone and with ozonation. The ultrasonic irradiation treatment was applied at three different frequencies (575, 861, and 1141 kHz). Due to the synergistic impact, the combination of ultrasound and ozone gave rise to a rate constant of almost 2.5 min−1, which was 625 times more than alone ultrasound. This hybrid degradation pathway, which is considered an advanced oxidative process, confirms the lower toxicity due to the low producing toxic intermediates [160].
Serna-Galvis et al. demonstrated the total elimination and mineralization of the antidepressant drug fluoxetine by sonochemical process combined with biological treatment. The alone biological treatment cannot remove the contaminant, even after five days and under desirable conditions. But, the sonochemical process (600 kHz) was able to eliminate the pharmaceutical. After 240 and 360 min of sonicating in the biological procedure, microorganisms could remove 20 and 70% of the primary total organic carbon, respectively [88].
3.2.3. Biological treatments
Biological treatment is a common method, especially for detergents and pharmaceutical pollutants that exist in wastewater. The effluent of this method can be returned to the river, and the sludge can be used as a fertilizer for the soil. The mentioned contamination can be considered as a nutrient source or energy for microorganisms in the biodegradation process. The mechanism of biodegradation can be changed by several factors such as anaerobic and aerobic conditions or the chemical structure of the contamination [[161], [162], [163]]. Aerobic degradation can be accomplished in various ways, such as using activated sludge tanks, oxidation bonds, and trickling filters. Each method has a considerable impact on removal efficiency. Although activated sludge tanks have almost 99% removal efficiency for LAS, however, the trickling filters have a wider range of 89.1–99.1% removal efficiencies [161,164]. Moreover, LAS has removal efficiency in the range of 40–85% under anaerobic conditions depending on which method was used [165,166]. In addition, the high chemical oxygen demand (COD) concentration of pharmaceutical wastewater caused it suitable for anaerobic processes. Based on some studies, COD reduction on antibiotic residues was by 70 to 75%, and some research reports that combining a filter and the anaerobic sludge blanket leads to high removal efficiency [167,168].
However, there are many limitations that cause it unsuitable to consider as a sole treatment method for the removal of surfactants from wastewater. One disadvantage is that this method is suitable for a low concentration of surfactant. This is because, in a concentration above almost 1,000,000 μg/L, the surfactant can depolarize the bacterial cell and can destroy function and structure [164,169]. By taking advantage of chemical pre-treatment for wastewater before enters to the treatment plant, mentioned problems could be mitigated. The huge advantage of pre-treatment of wastewater is to convert these compounds to products that could be more biodegraded. There are numerous methods for per-treatment, and AOPs can be considered as one of the commonly used methods [164,169].
3.2.4. Coagulation and flocculation
Coagulation-flocculation is considered a chemical water treatment method that can usually be applied prior to filtration and sedimentation. This simple and low-cost method is used to improve the ability of a treatment action in order to remove pollutants such as surfactants. Coagulation can form a mass which is large enough to be trapped or settle in the filter. Flocculation is considered as gentle agitation or stirring to reinforce the particles formed to be clustered into large masses to settle from solution. Inorganic coagulants (Polymer-type) such as Fe2(SO4)3, FeSO4·7H2O, and Al2(SO4)3·18H2O are generally used in this process [170,171]. Residual contents of inorganic coagulants like Al2(SO4)3·18H2O might cause Alzheimer's disease [172]. Thus, it is better to use fewer inorganic coagulants, and these materials should be replaced with ecofriendly materials [173]. It has been turned out that this method is effective and useful for the removal of the surfactant from wastewaters. In many kinds of research, removing the surfactant was roughly 95% in wastewater, and COD reduction was approximately 88% [147].
4. Conclusion and future perspective
Presently, there is a considerable research gap in the survival of SARS-CoV 2 in the water and the potential of coronavirus transmission by untreated and treated wastewater. The excretion of the virus via feces is quite obvious from recent researches. Hence, this research investigates the occurrence, detection, and recommended removal treatment ways of coronavirus in wastewater with different techniques commonly used at the different stages of the treatment, including primary, secondary, and tertiary phases in the wastewater treatment plants. The research of Nasseri et al. is a practical example of using the proposed methods to inactivate the SARS-CoV-2 in WWTP. They evaluated the presence of SARS-CoV 2 in the outlet samples from ultraviolet (UV) disinfection (module outlet 5 to 6) and samples from chlorine disinfection (module outlet 1 to 4) of WWTPs in southern Tehran, Iran, and the results showed that only chlorine disinfection samples remain positive. Hence, the UV disinfection was more successful than chlorine disinfection, and operators of WWTPs should enhance the free residual chlorine concentration to ≥0.5 mg·L−1 for effective disinfection [192]. The development of environmentally friendly and low-cost processes for viral removal are other opportunities for scholars to manage and reduce the growing health and environmental risks from any similar future crisis.
Another crucial environmental concern related to the COVID-19 epidemic is the extra use of disinfectants, detergents, and soaps and the overuse of specific drugs to protect against virus or novel mental problems related to quarantine. Their excretion into wastewater not only can pollute drinking water resources but also includes a potential threat to the aquatic environment. Hence, future studies should be directed to update and re-design WWTP to remove these extra amounts of organic compounds efficiently and create environmentally-friendly cleaning products such as hand sanitizers and soaps with low toxic by-product formation for humans and environmental safety.
Further communication among regulatory agencies with chemical, biological and medical researchers is needed to explore additional acceptance and validation of these water treatment approaches, which are applied to make claims of successful water treatment, especially during the COVID-19 crisis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to gratefully thank the Amirkabir University of Technology (AUT), Tehran, Iran, for their financial support.
References
- 1.Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lesimple A., Jasim S.Y., Johnson D.J., Hilal N. The role of wastewater treatment plants as tools for SARS-CoV-2 early detection and removal. J. Water Process Eng. 2020:101544. doi: 10.1016/j.jwpe.2020.101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrillo-Reyes J., Barragán-Trinidad M., Buitrón G. Surveillance of SARS-CoV-2 in sewage and wastewater treatment plants in Mexico. J. Water Process Eng. 2020:101815. doi: 10.1016/j.jwpe.2020.101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cervantes-Avilés P., Moreno-Andrade I., Carrillo-Reyes J. Approaches applied to detect SARS-CoV-2 in wastewater and perspectives post-COVID-19. J. Water Process Eng. 2021:101947. doi: 10.1016/j.jwpe.2021.101947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teymourian T., Teymoorian T., Kowsari E., Ramakrishna S. Challenges, strategies, and recommendations for the huge surge in plastic and medical waste during the global COVID-19 pandemic with circular economy approach. Mater. Circ. Econ. 2021;3(1):1–14. [Google Scholar]
- 6.Parsa S.M. Reliability of thermal desalination (solar stills) for water/wastewater treatment in light of COVID-19 (novel coronavirus “SARS-CoV-2”) pandemic: what should consider? Desalination. 2021:115106. doi: 10.1016/j.desal.2021.115106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abrams H.R., Loomer L., Gandhi A., Grabowski D.C. Characteristics of US nursing homes with COVID-19 cases. J. Am. Geriatr. Soc. 2020;68(8):1653–1656. doi: 10.1111/jgs.16661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franco-Paredes C., Jankousky K., Schultz J., Bernfeld J., Cullen K., Quan N.G., Kon S., Hotez P., Henao-Martínez A.F., Krsak M. COVID-19 in jails and prisons: a neglected infection in a marginalized population. PLoS Negl. Trop. Dis. 2020;14(6):e0008409. doi: 10.1371/journal.pntd.0008409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waltenburg M.A., Victoroff T., Rose C.E., Butterfield M., Jervis R.H., Fedak K.M., Gabel J.A., Feldpausch A., Dunne E.M., Austin C. Update: COVID-19 among workers in meat and poultry processing facilities—United States, April–May 2020. Morb. Mortal. Wkly Rep. 2020;69(27):887. doi: 10.15585/mmwr.mm6927e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Organization, W.H . World Health Organization; 2020. Modes of Transmission of Virus Causing COVID-19: Implications for IPC Precaution Recommendations: Scientific Brief, 27 March 2020. [Google Scholar]
- 11.Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quilliam R.S., Weidmann M., Moresco V., Purshouse H., O’Hara Z., Oliver D.M. COVID-19: the environmental implications of shedding SARS-CoV-2 in human faeces. Environ. Int. 2020;140:105790. doi: 10.1016/j.envint.2020.105790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart O.E., Halden R.U. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: feasibility, economy, opportunities and challenges. Sci. Total Environ. 2020:138875. doi: 10.1016/j.scitotenv.2020.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. Jama. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020:139076. doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castillo-Zacarías C., Barocio M.E., Hidalgo-Vázquez E., Sosa-Hernández J.E., Parra-Arroyo L., López-Pacheco I.Y., Barceló D., Iqbal H.N., Parra-Saldívar R. Antidepressant drugs as emerging contaminants: occurrence in urban and non-urban waters and analytical methods for their detection. Sci. Total Environ. 2020:143722. doi: 10.1016/j.scitotenv.2020.143722. [DOI] [PubMed] [Google Scholar]
- 17.Abelenda-Alonso G., Padullés A., Rombauts A., Gudiol C., Pujol M., Alvarez-Pouso C., Jodar R., Carratalà J. Antibiotic prescription during the COVID-19 pandemic: a biphasic pattern. Infect. Control Hosp. Epidemiol. 2020;41(11):1371–1372. doi: 10.1017/ice.2020.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S., Green H.C., Wilder M.L., Du Q., Kmush B.L., Collins M.B., Larsen D.A., Zeng T. High-throughput wastewater analysis for substance use assessment in central New York during the COVID-19 pandemic. Environ Sci Process Impacts. 2020;22(11):2147–2161. doi: 10.1039/d0em00377h. [DOI] [PubMed] [Google Scholar]
- 19.Kweinor Tetteh E., Opoku Amankwa M., Armah E.K., Rathilal S. Fate of COVID-19 occurrences in wastewater systems: emerging detection and treatment technologies—a review. Water. 2020;12(10):2680. [Google Scholar]
- 20.Gu J., Han B., Wang J. COVID-19: gastrointestinal manifestations and potential fecal–oral transmission. Gastroenterology. 2020;158(6):1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price E. Could the severity of COVID-19 be increased by low gastric acidity? Crit. Care. 2020;24(1):1–2. doi: 10.1186/s13054-020-03182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO, U . 2017. WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation. [Google Scholar]
- 23.Leung W.K., To K.-f, Chan P.K., Chan H.L., Wu A.K., Lee N., Yuen K.Y., Sung J.J. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125(4):1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A., Flemban H., Al-Nassir W.N., Balkhy H.H., Al-Hakeem R.F. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect. Dis. 2013;13(9):752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corman V.M., Albarrak A.M., Omrani A.S., Albarrak M.M., Farah M.E., Almasri M., Muth D., Sieberg A., Meyer B., Assiri A.M. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin. Infect. Dis. 2016;62(4):477–483. doi: 10.1093/cid/civ951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J., Li C., Zhao G., Chu H., Wang D., Yan H.H.-N., Poon V.K.-M., Wen L., Wong B.H.-Y., Zhao X. Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci. Adv. 2017;3(11):eaao4966. doi: 10.1126/sciadv.aao4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lescure F.-X., Bouadma L., Nguyen D., Parisey M., Wicky P.-H., Behillil S., Gaymard A., Bouscambert-Duchamp M., Donati F., Le Hingrat Q. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect. Dis. 2020;20(6):697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang A., Tong Z.-D., Wang H.-L., Dai Y.-X., Li K.-F., Liu J.-N., Wu W.-J., Yuan C., Yu M.-L., Li P. 2020. Early Release-Detection of Novel Coronavirus by RT-PCR in Stool Specimen From Asymptomatic Child, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saawarn B., Hait S. Occurrence, fate and removal of SARS-CoV-2 in wastewater: current knowledge and future perspectives. J. Environ. Chem. Eng. 2020:104870. doi: 10.1016/j.jece.2020.104870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.UNICEF https://www.unicef.org/wash/files/UNICEF_Game_plan_to_end_open_defecation_2018.pdf [2018 22.6.2020]; Available from:
- 31.Kataki S., Chatterjee S., Vairale M.G., Sharma S., Dwivedi S.K. Concerns and strategies for wastewater treatment during COVID-19 pandemic to stop plausible transmission. Resour. Conserv. Recycl. 2020:105156. doi: 10.1016/j.resconrec.2020.105156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anayah F., Al-Khatib I.A., Hejaz B. Assessment of water and sanitation systems at Palestinian healthcare facilities: pre-and post-COVID-19. Environ. Monit. Assess. 2021;193(1):1–22. doi: 10.1007/s10661-020-08791-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sunkari E.D., Korboe H.M., Abu M., Kizildeniz T. Sources and routes of SARS-CoV-2 transmission in water systems in Africa: are there any sustainable remedies? Sci. Total Environ. 2020:142298. doi: 10.1016/j.scitotenv.2020.142298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.La Rosa G., Iaconelli M., Mancini P., Ferraro G.B., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherchan S.P., Shahin S., Ward L.M., Tandukar S., Aw T.G., Schmitz B., Ahmed W., Kitajima M. First detection of SARS-CoV-2 RNA in wastewater in North America: a study in Louisiana, USA. Sci. Total Environ. 2020;743:140621. doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green H., Wilder M., Middleton F.A., Collins M., Fenty A., Gentile K., Kmush B., Zeng T., Larsen D.A. Quantification of SARS-CoV-2 and cross-assembly phage (crAssphage) from wastewater to monitor coronavirus transmission within communities. MedRxiv. 2020 doi: 10.1101/2020.05.21.20109181. 2020.05.21.20109181. [DOI] [Google Scholar]
- 37.Kocamemi B.A., Kurt H., Sait A., Sarac F., Saatci A.M., Pakdemirli B. SARS-CoV-2 detection in Istanbul wastewater treatment plant sludges. medRxiv. 2020 doi: 10.1101/2020.05.12.20099358. 2020.05.12.20099358. [DOI] [Google Scholar]
- 38.Kocamemi B.A., Kurt H., Hacioglu S., Yarali C., Saatci A.M., Pakdemirli B. First data-set on SARS-CoV-2 detection for Istanbul wastewaters in Turkey. MedRxiv. 2020 doi: 10.1101/2020.05.12.20099358. 2020.05.03.20089417. [DOI] [Google Scholar]
- 39.Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanhaei M., Mohebbi S.R., Hosseini S.M., Rafieepoor M., Kazemian S., Ghaemi A., Shamloei S., Mirjalali H., Aghdaei H.A., Zali M.R. The first detection of SARS-CoV-2 RNA in the wastewater of Tehran, Iran. Environ. Sci. Pollut. Res. 2021:1–8. doi: 10.1007/s11356-021-13393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chin A., Chu J., Perera M., Hui K., Yen H.-L., Chan M., Peiris M., Poon L. Stability of SARS-CoV-2 in different environmental conditions. MedRxiv. 2020 doi: 10.1016/S2666-5247(20)30003-3. 2020.03.15.20036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgan W. The Conversation. 2020. Coronavirus: heavy use of hand sanitisers could boost antimicrobial resistance. [Google Scholar]
- 45.Klemeš J.J., Van Fan Y., Jiang P. The energy and environmental footprints of COVID-19 fighting measures–PPE, disinfection, supply chains. Energy. 2020;211:118701. doi: 10.1016/j.energy.2020.118701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Juela Quintuña D.M. 2020. Estimated Impact of COVID-19 on Water Needs and Volume and Quality of Wastewater. Available at SSRN 3651551. [Google Scholar]
- 47.Ihsanullah I., Bilal M., Naushad M. Coronavirus 2 (SARS-CoV-2) in water environments: current status, challenges and research opportunities. J. Water Process Eng. 2021;39:101735. doi: 10.1016/j.jwpe.2020.101735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daverey A., Dutta K. COVID-19: eco-friendly hand hygiene for human and environmental safety. J. Environ. Chem. Eng. 2020:104754. doi: 10.1016/j.jece.2020.104754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Organization, W.H . World Health Organization; 2020. Water, Sanitation, Hygiene and Waste Management for COVID-19: Technical Brief, 03 March 2020. [Google Scholar]
- 50.Sturman L.S., Ricard C., Holmes K. Conformational change of the coronavirus peplomer glycoprotein at pH 8.0 and 37 degrees C correlates with virus aggregation and virus-induced cell fusion. J. Virol. 1990;64(6):3042–3050. doi: 10.1128/jvi.64.6.3042-3050.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pramod K., Kotta S., Jijith U., Aravind A., Tahir M.A., Manju C., Gangadharappa H. Surfactant-based prophylaxis and therapy against COVID-19: a possibility. Med. Hypotheses. 2020;143:110081. doi: 10.1016/j.mehy.2020.110081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiel-Shafran A., Ronen Z., Weisbrod N., Adar E., Gross A. Potential changes in soil properties following irrigation with surfactant-rich greywater. Ecol. Eng. 2006;26(4):348–354. [Google Scholar]
- 53.Sawadogo B., Sou M., Hijikata N., Sangare D., Maiga A.H., Funamizu N. Effect of detergents from grey water on irrigated plants: case of okra (Abelmoschus esculentus) and lettuce (Lactuca sativa) J. Arid Land Stud. 2014;24(1):117–120. [Google Scholar]
- 54.Chaturvedi A.D., Tiwari K. Effect of household detergents (surfactants) degraded through aquatic fungi. Recent Res. Sci. Technol. 2013;5(5) [Google Scholar]
- 55.Ríos F., Lechuga M., Fernández-Serrano M., Fernández-Arteaga A. Aerobic biodegradation of amphoteric amine-oxide-based surfactants: effect of molecular structure, initial surfactant concentration and pH. Chemosphere. 2017;171:324–331. doi: 10.1016/j.chemosphere.2016.12.070. [DOI] [PubMed] [Google Scholar]
- 56.Mousavi S.A., Khodadoost F. Effects of detergents on natural ecosystems and wastewater treatment processes: a review. Environ. Sci. Pollut. Res. 2019;26(26):26439–26448. doi: 10.1007/s11356-019-05802-x. [DOI] [PubMed] [Google Scholar]
- 57.Meconi G.M., Ballard N., Asua J.M., Zangi R. Adsorption and desorption behavior of ionic and nonionic surfactants on polymer surfaces. Soft Matter. 2016;12(48):9692–9704. doi: 10.1039/c6sm01878e. [DOI] [PubMed] [Google Scholar]
- 58.Lee K., Park K., Khanal S., Lee J. Effects of household detergent on anaerobic fermentation of kitchen wastewater from food waste disposer. J. Hazard. Mater. 2013;244:39–45. doi: 10.1016/j.jhazmat.2012.10.073. [DOI] [PubMed] [Google Scholar]
- 59.Ivanković T., Hrenović J. Surfactants in the environment. Arch. Ind. Hyg. Toxicol. 2010;61(1):95–110. doi: 10.2478/10004-1254-61-2010-1943. [DOI] [PubMed] [Google Scholar]
- 60.Clara M., Scharf S., Scheffknecht C., Gans O. Occurrence of selected surfactants in untreated and treated sewage. Water Res. 2007;41(19):4339–4348. doi: 10.1016/j.watres.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 61.Information, N.C.f.B PubChem Compound Summary. https://pubchem.ncbi.nlm.nih.gov [May 4, 2021]; Available from:
- 62.Negin C., Ali S., Xie Q. Most common surfactants employed in chemical enhanced oil recovery. Petroleum. 2017;3(2):197–211. [Google Scholar]
- 63.Rajan D.S. An evaluation of the effect of a detergent on dissolved oxygen consumption rate of Anabas testudineus. Int. J. Fish. Aquat. Stud. 2015;2(6):46–48. [Google Scholar]
- 64.Pattusamy V., Nandini N. Detergent and sewage phosphates entering into lake ecosystem and its impact on aquatic environment. Int. J. Adv. Res. 2013;1(3) [Google Scholar]
- 65.Rejeki S., Desrina D., Mulyana A.R. Chronic affects of detergent surfactant (linear alkylbenzene sulfonate/LAS) on the growth and survival rate of sea bass (Lates calcalifer Bloch) larvae. J. Coast. Develop. 2008 [Google Scholar]
- 66.Peng Z., Darnault C.J., Tian F., Baveye P.C., Hu H. Influence of anionic surfactant on saturated hydraulic conductivity of loamy sand and sandy loam soils. Water. 2017;9(6):433. [Google Scholar]
- 67.Jing J.L.J., Pei Yi T., Bose R.J., McCarthy J.R., Tharmalingam N., Madheswaran T. Hand sanitizers: a review on formulation aspects, adverse effects, and regulations. Int. J. Environ. Res. Public Health. 2020;17(9):3326. doi: 10.3390/ijerph17093326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slaughter R., Mason R., Beasley D., Vale J., Schep L. Isopropanol poisoning. Clin. Toxicol. 2014;52(5):470–478. doi: 10.3109/15563650.2014.914527. [DOI] [PubMed] [Google Scholar]
- 69.Mahmood A., Eqan M., Pervez S., Alghamdi H.A., Tabinda A.B., Yasar A., Brindhadevi K., Pugazhendhi A. COVID-19 and frequent use of hand sanitizers; human health and environmental hazards by exposure pathways. Sci. Total Environ. 2020;742:140561. doi: 10.1016/j.scitotenv.2020.140561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malik M., Tahir M.J., Jabbar R., Ahmed A., Hussain R. Self-medication during Covid-19 pandemic: challenges and opportunities. Drugs Ther. Perspect. 2020;36(12):565–567. doi: 10.1007/s40267-020-00785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.da Silva C.F., Deutschendorf C., Nagel F.M., Dalmora C.H., Dos Santos R.P., Lisboa T.C. Impact of the pandemic on antimicrobial consumption patterns. Infect. Control Hosp. Epidemiol. 2020:1–3. doi: 10.1017/ice.2020.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nason S., Lin E., Eitzer B.D., Koelmel J.P., Peccia J. 2021. Traffic, Drugs, Mental Health, and Disinfectants: Changes in Sewage Sludge Chemical Signatures During a COVID-19 Community Lockdown. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rawson T.M., Moore L.S., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., Satta G., Cooke G., Holmes A. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020;71(9):2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Usman M., Farooq M., Hanna K. Environmental side effects of the injudicious use of antimicrobials in the era of COVID-19. Sci. Total Environ. 2020;745:141053. doi: 10.1016/j.scitotenv.2020.141053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Getahun H., Smith I., Trivedi K., Paulin S., Balkhy H.H. Tackling antimicrobial resistance in the COVID-19 pandemic. Bull. World Health Organ. 2020;98(7):442. doi: 10.2471/BLT.20.268573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anastopoulos I., Pashalidis I., Orfanos A.G., Manariotis I.D., Tatarchuk T., Sellaoui L., Bonilla-Petriciolet A., Mittal A., Núñez-Delgado A. Removal of caffeine, nicotine and amoxicillin from (waste) waters by various adsorbents. A review. J. Environ. Manag. 2020;261:110236. doi: 10.1016/j.jenvman.2020.110236. [DOI] [PubMed] [Google Scholar]
- 77.Cohen J. American Association for the Advancement of Science; 2018. ‘Frightening’ Typhoid Fever Outbreak Spreads in Pakistan. [DOI] [PubMed] [Google Scholar]
- 78.Ozamiz-Etxebarria N., Dosil-Santamaria M., Picaza-Gorrochategui M., Idoiaga-Mondragon N. Stress, anxiety, and depression levels in the initial stage of the COVID-19 outbreak in a population sample in the northern Spain. Cadernos Saude Publ. 2020;36:e00054020. doi: 10.1590/0102-311X00054020. [DOI] [PubMed] [Google Scholar]
- 79.Smith L., Jacob L., Yakkundi A., McDermott D., Armstrong N.C., Barnett Y., López-Sánchez G.F., Martin S., Butler L., Tully M.A. Correlates of symptoms of anxiety and depression and mental wellbeing associated with COVID-19: a cross-sectional study of UK-based respondents. Psychiatry Res. 2020;291:113138. doi: 10.1016/j.psychres.2020.113138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Z., Gao S., Dai Q., Zhao M., Yang F. Occurrence and risk assessment of psychoactive substances in tap water from China. Environ. Pollut. 2020;261:114163. doi: 10.1016/j.envpol.2020.114163. [DOI] [PubMed] [Google Scholar]
- 81.OECD . OECD Health Statistics (Database) 2020. OECD health data: pharmaceutical market. [Google Scholar]
- 82.Thiebault T., Boussafir M. Adsorption mechanisms of psychoactive drugs onto montmorillonite. Colloid Interface Sci. Commun. 2019;30:100183. [Google Scholar]
- 83.Deng Y., Ok Y.S., Mohan D., Pittman C.U., Jr., Dou X. Carbamazepine removal from water by carbon dot-modified magnetic carbon nanotubes. Environ. Res. 2019;169:434–444. doi: 10.1016/j.envres.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 84.Rasheed T., Bilal M., Hassan A.A., Nabeel F., Bharagava R.N., Ferreira L.F.R., Tran H.N., Iqbal H.M. Environmental threatening concern and efficient removal of pharmaceutically active compounds using metal-organic frameworks as adsorbents. Environ. Res. 2020;185:109436. doi: 10.1016/j.envres.2020.109436. [DOI] [PubMed] [Google Scholar]
- 85.Neuparth T., Lopes A.I., Alves N., Oliveira J.M., Santos M.M. Does the antidepressant sertraline show chronic effects on aquatic invertebrates at environmentally relevant concentrations? A case study with the keystone amphipod, Gammarus locusta. Ecotoxicol. Environ. Saf. 2019;183:109486. doi: 10.1016/j.ecoenv.2019.109486. [DOI] [PubMed] [Google Scholar]
- 86.Fong P.P., Hoy C.M. Antidepressants (venlafaxine and citalopram) cause foot detachment from the substrate in freshwater snails at environmentally relevant concentrations. Mar. Freshw. Behav. Physiol. 2012;45(2):145–153. [Google Scholar]
- 87.Ferrari B.t., Paxéus N., Giudice R.L., Pollio A., Garric J. Ecotoxicological impact of pharmaceuticals found in treated wastewaters: study of carbamazepine, clofibric acid, and diclofenac. Ecotoxicol. Environ. Saf. 2003;55(3):359–370. doi: 10.1016/s0147-6513(02)00082-9. [DOI] [PubMed] [Google Scholar]
- 88.Serna-Galvis E.A., Silva-Agredo J., Giraldo-Aguirre A.L., Torres-Palma R.A. Sonochemical degradation of the pharmaceutical fluoxetine: effect of parameters, organic and inorganic additives and combination with a biological system. Sci. Total Environ. 2015;524:354–360. doi: 10.1016/j.scitotenv.2015.04.053. [DOI] [PubMed] [Google Scholar]
- 89.Ab Rahman M.F., Rusli A., Misman M.A., Rashid A.A. Biodegradable gloves for waste management post-COVID-19 outbreak: a shelf-life prediction. ACS Omega. 2020;5(46):30329–30335. doi: 10.1021/acsomega.0c04964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baquero F., Martínez J.-L., Cantón R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008;19(3):260–265. doi: 10.1016/j.copbio.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 91.Metcalf L., Eddy H.P., Tchobanoglous G. vol. 4. McGraw-Hill New York; 1991. Wastewater Engineering: Treatment, Disposal, and Reuse. [Google Scholar]
- 92.Gerba C.P., Pepper I.L. Environmental and Pollution Science. Elsevier; 2019. Municipal wastewater treatment; pp. 393–418. [Google Scholar]
- 93.Vickers N.J. Animal communication: when I’m calling you, will you answer too? Curr. Biol. 2017;27(14):R713–R715. doi: 10.1016/j.cub.2017.05.064. [DOI] [PubMed] [Google Scholar]
- 94.Randazzo W., Cuevas-Ferrando E., Sanjuán R., Domingo-Calap P., Sánchez G. Metropolitan wastewater analysis for COVID-19 epidemiological surveillance. Int. J. Hyg. Environ. Health. 2020;230:113621. doi: 10.1016/j.ijheh.2020.113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang X.-W., Li J.-S., Jin M., Zhen B., Kong Q.-X., Song N., Xiao W.-J., Yin J., Wei W., Wang G.-J. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol. Methods. 2005;126(1–2):171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang X.-W., Li J.-S., Guo T.-K., Zhen B., Kong Q.-X., Yi B., Li Z., Song N., Jin M., Xiao W.-J. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan Hospital and the 309th Hospital. J. Virol. Methods. 2005;128(1–2):156–161. doi: 10.1016/j.jviromet.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Clarke N.A., Stevenson R.E., Chang S.L., Kabler P.W. Removal of enteric viruses from sewage by activated sludge treatment. Am. J. Publ. Health Nations Health. 1961;51(8):1118–1129. doi: 10.2105/ajph.51.8.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Obotey Ezugbe E., Rathilal S. Membrane technologies in wastewater treatment: a review. Membranes. 2020;10(5):89. doi: 10.3390/membranes10050089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jung Y.H., Kim I.J., Han J.-I., Choi I.-G., Kim K.H. Aqueous ammonia pretreatment of oil palm empty fruit bunches for ethanol production. Bioresour. Technol. 2011;102(20):9806–9809. doi: 10.1016/j.biortech.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 100.Wang J., Shen J., Ye D., Yan X., Zhang Y., Yang W., Li X., Wang J., Zhang L., Pan L. Disinfection technology of hospital wastes and wastewater: suggestions for disinfection strategy during coronavirus disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2020:114665. doi: 10.1016/j.envpol.2020.114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen C., Zhang X.-J., Wang Y., Zhu L.-X., Liu J. Waste water disinfection during SARS epidemic for mecrobiological and toxicological control. Biomed. Environ. Sci. 2006;19(3):173. [PubMed] [Google Scholar]
- 102.Xiao K., Liang S., Wang X., Chen C., Huang X. Current state and challenges of full-scale membrane bioreactor applications: a critical review. Bioresour. Technol. 2019;271:473–481. doi: 10.1016/j.biortech.2018.09.061. [DOI] [PubMed] [Google Scholar]
- 103.Simmons F.J., Kuo D.H.-W., Xagoraraki I. Removal of human enteric viruses by a full-scale membrane bioreactor during municipal wastewater processing. Water Res. 2011;45(9):2739–2750. doi: 10.1016/j.watres.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 104.Ye Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ. Sci. Technol. 2016;50(10):5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- 105.Pinon A., Vialette M. Survival of viruses in water. Intervirology. 2018;61(5):214–222. doi: 10.1159/000484899. [DOI] [PubMed] [Google Scholar]
- 106.Bivins A., Greaves J., Fischer R., Yinda K.C., Ahmed W., Kitajima M., Munster V.J., Bibby K. Persistence of SARS-CoV-2 in water and wastewater. Environ. Sci. Technol. Lett. 2020;7(12):937–942. doi: 10.1021/acs.estlett.0c00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ojha A. Nanomaterials for removal of waterborne pathogens: opportunities and challenges. Waterborne Pathogens. 2020:385–432. [Google Scholar]
- 108.Indarto A., Ikhsan N.A., Wibowo I. Applications of carbon nanotubes for controlling waterborne pathogens. Waterborne Pathogens. 2020:433–461. [Google Scholar]
- 109.Fitzgibbon J., Sagripanti J.L. Analysis of the survival of Venezuelan equine encephalomyelitis virus and possible viral simulants in liquid suspensions. J. Appl. Microbiol. 2008;105(5):1477–1483. doi: 10.1111/j.1365-2672.2008.03919.x. [DOI] [PubMed] [Google Scholar]
- 110.Kuznesof P. Chemical and Technical Assessment 61st JECFA FAO. 2004. Sodium dichloroisocyanurate (NaDCC–anhydrous and dihydrate) [Google Scholar]
- 111.Pinto G., Rohrig B. Use of chloroisocyanuarates for disinfection of water: application of miscellaneous general chemistry topics. J. Chem. Educ. 2003;80(1):41. [Google Scholar]
- 112.Bruins G., Dyer J. Environmental considerations of disinfectants used in agriculture. Rev. Sci. Techn. (Int. Off. Epizootics) 1995;14(1):81–94. doi: 10.20506/rst.14.1.826. [DOI] [PubMed] [Google Scholar]
- 113.Bull R.J., Gerba C., Trussell R.R. Evaluation of the health risks associated with disinfection. Crit. Rev. Environ. Sci. Technol. 1990;20(2):77–113. [Google Scholar]
- 114.Zhang D., Ling H., Huang X., Li J., Li W., Yi C., Zhang T., Jiang Y., He Y., Deng S. Potential spreading risks and disinfection challenges of medical wastewater by the presence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of Fangcang Hospital. Sci. Total Environ. 2020;741:140445. doi: 10.1016/j.scitotenv.2020.140445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lasik M., Dobrucka R., Konieczny P. Impedimetric test for rapid determination of performic acid (PFA) biocidal activity toward Echerichia coli. Acta Sci. Pol. Technol. Aliment. 2013;12(4) [Google Scholar]
- 116.Luukkonen T., Heyninck T., Rämö J., Lassi U. Comparison of organic peracids in wastewater treatment: disinfection, oxidation and corrosion. Water Res. 2015;85:275–285. doi: 10.1016/j.watres.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 117.HEINONEN-TANSKI H., Miettinen H. Performic acid as a potential disinfectant at low temperature. J. Food Process Eng. 2010;33(6):1159–1172. [Google Scholar]
- 118.Gehr R., Chen D., Moreau M. Performic acid (PFA): tests on an advanced primary effluent show promising disinfection performance. Water Sci. Technol. 2009;59(1):89–96. doi: 10.2166/wst.2009.761. [DOI] [PubMed] [Google Scholar]
- 119.Raeiszadeh M., Adeli B. A critical review on ultraviolet disinfection systems against COVID-19 outbreak: applicability, validation, and safety considerations. ACS Photonics. 2020;7(11):2941–2951. doi: 10.1021/acsphotonics.0c01245. [DOI] [PubMed] [Google Scholar]
- 120.Hijnen W., Beerendonk E., Medema G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo) cysts in water: a review. Water Res. 2006;40(1):3–22. doi: 10.1016/j.watres.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 121.Wigginton K.R., Kohn T. Virus disinfection mechanisms: the role of virus composition, structure, and function. Curr. Opin. Virol. 2012;2(1):84–89. doi: 10.1016/j.coviro.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Darnell M.E., Subbarao K., Feinstone S.M., Taylor D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J. Virol. Methods. 2004;121(1):85–91. doi: 10.1016/j.jviromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Malley J., Jr., Burris B. World Water Congress, Orlando, FL. 2001. Bridging the gap: meeting the world’s water and environmental resources challenges. [Google Scholar]
- 124.Morrison C., Atkinson A., Zamyadi A., Kibuye F., McKie M., Hogard S., Mollica P., Jasim S., Wert E.C. Critical review and research needs of ozone applications related to virus inactivation: potential implications for SARS-CoV-2. Ozone Sci. Eng. 2021;43(1):2–20. [Google Scholar]
- 125.Tizaoui C. Ozone: a potential oxidant for COVID-19 virus (SARS-CoV-2) Ozone Sci. Eng. 2020;42(5):378–385. [Google Scholar]
- 126.Martins R.B., Castro I.A., Pontelli M., Souza J.P., Lima T.M., Melo S.R., Siqueira J.P.Z., Caetano M.H., Arruda E., de Almeida M.T.G. SARS-CoV-2 inactivation by ozonated water: a preliminary alternative for environmental disinfection. Ozone Sci. Eng. 2020:1–4. [Google Scholar]
- 127.Koivunen J., Heinonen-Tanski H. Peracetic acid (PAA) disinfection of primary, secondary and tertiary treated municipal wastewaters. Water Res. 2005;39(18):4445–4453. doi: 10.1016/j.watres.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 128.Petrović M., Hernando M.D., Díaz-Cruz M.S., Barceló D. Liquid chromatography–tandem mass spectrometry for the analysis of pharmaceutical residues in environmental samples: a review. J. Chromatogr. A. 2005;1067(1–2):1–14. doi: 10.1016/j.chroma.2004.10.110. [DOI] [PubMed] [Google Scholar]
- 129.Rivera-Utrilla J., Sánchez-Polo M., Ferro-García M.Á., Prados-Joya G., Ocampo-Pérez R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere. 2013;93(7):1268–1287. doi: 10.1016/j.chemosphere.2013.07.059. [DOI] [PubMed] [Google Scholar]
- 130.Tong A.Y., Peake B.M., Braund R. Disposal practices for unused medications around the world. Environ. Int. 2011;37(1):292–298. doi: 10.1016/j.envint.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 131.Kariim I., Abdulkareem A., Abubakre O. Development and characterization of MWCNTs from activated carbon as adsorbent for metronidazole and levofloxacin sorption from pharmaceutical wastewater: kinetics, isotherms and thermodynamic studies. Sci. Afr. 2020;7:e00242. [Google Scholar]
- 132.Hosseinnia A., Hashtroudi M., Pazouki M., Banifatemi M. Removal of surfactants from wastewater by rice husk. Iran. J. Chem. Eng. 2006;3(3):44–50. [Google Scholar]
- 133.Kibbey T.C., Paruchuri R., Sabatini D.A., Chen L. Adsorption of beta blockers to environmental surfaces. Environ. Sci. Technol. 2007;41(15):5349–5356. doi: 10.1021/es070152v. [DOI] [PubMed] [Google Scholar]
- 134.Ncibi M.C., Gaspard S., Sillanpää M. As-synthesized multi-walled carbon nanotubes for the removal of ionic and non-ionic surfactants. J. Hazard. Mater. 2015;286:195–203. doi: 10.1016/j.jhazmat.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 135.Jung C., Son A., Her N., Zoh K.-D., Cho J., Yoon Y. Removal of endocrine disrupting compounds, pharmaceuticals, and personal care products in water using carbon nanotubes: a review. J. Ind. Eng. Chem. 2015;27:1–11. [Google Scholar]
- 136.Delgado N., Capparelli A., Navarro A., Marino D. Pharmaceutical emerging pollutants removal from water using powdered activated carbon: study of kinetics and adsorption equilibrium. J. Environ. Manag. 2019;236:301–308. doi: 10.1016/j.jenvman.2019.01.116. [DOI] [PubMed] [Google Scholar]
- 137.Aljeboree A.M., Alshirifi A.N. Adsorption of pharmaceuticals as emerging contaminants from aqueous solutions on to friendly surfaces such as activated carbon: a review. J. Pharm. Sci. Res. 2018;10(9):2252–2257. [Google Scholar]
- 138.Rosu M., Marlina A., Kaya A., Schumpe A. Surfactant adsorption onto activated carbon and its effect on absorption with chemical reaction. Chem. Eng. Sci. 2007;62(24):7336–7343. [Google Scholar]
- 139.Schouten N., van der Ham L.G., Euverink G.-J.W., de Haan A.B. Selection and evaluation of adsorbents for the removal of anionic surfactants from laundry rinsing water. Water Res. 2007;41(18):4233–4241. doi: 10.1016/j.watres.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 140.Bindes M.M., Franco M.R., Jr. Surfactant removal from aqueous solutions onto activated carbon using UV spectroscopy. Desalin. Water Treat. 2015;56(11):2890–2895. [Google Scholar]
- 141.Crini G., Badot P.-M. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: a review of recent literature. Prog. Polym. Sci. 2008;33(4):399–447. [Google Scholar]
- 142.Parhizgar F., Alishahi A., Varasteh H., Rezaee H. Removing sodium dodecyl benzene sulfonate (SDBS) from aqueous solutions using chitosan. J. Polym. Environ. 2017;25(3):836–843. [Google Scholar]
- 143.Siyal A.A., Shamsuddin M.R., Low A., Rabat N.E. A review on recent developments in the adsorption of surfactants from wastewater. J. Environ. Manag. 2020;254:109797. doi: 10.1016/j.jenvman.2019.109797. [DOI] [PubMed] [Google Scholar]
- 144.Ayoub K., van Hullebusch E.D., Cassir M., Bermond A. Application of advanced oxidation processes for TNT removal: a review. J. Hazard. Mater. 2010;178(1–3):10–28. doi: 10.1016/j.jhazmat.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 145.Esplugas S., Yue P., Pervez M.I. Degradation of 4-chlorophenol by photolytic oxidation. Water Res. 1994;28(6):1323–1328. [Google Scholar]
- 146.Munter R. Advanced oxidation processes–current status and prospects. Proc. Estonian Acad. Sci. Chem. 2001;50(2):59–80. [Google Scholar]
- 147.Krishnan S., Chandran K., Sinnathambi C.M. Wastewater treatment technologies used for the removal of different surfactants: a comparative. Int. J. Appl. Chem. 2016;12(4):727–739. [Google Scholar]
- 148.Hoigné J., Bader H. Rate constants of reactions of ozone with organic and inorganic compounds in water—II: dissociating organic compounds. Water Res. 1983;17(2):185–194. [Google Scholar]
- 149.Zhang Y., Han C., Zhang G., Dionysiou D.D., Nadagouda M.N. PEG-assisted synthesis of crystal TiO2 nanowires with high specific surface area for enhanced photocatalytic degradation of atrazine. Chem. Eng. J. 2015;268:170–179. [Google Scholar]
- 150.Yu Y.H., Su J.F., Shih Y., Wang J., Wang P.Y., Huang C.P. Hazardous wastes treatment technologies. Water Environ. Res. 2020;92(10):1833–1860. doi: 10.1002/wer.1447. [DOI] [PubMed] [Google Scholar]
- 151.Sharma S., Mukhopadhyay M., Murthy Z. Treatment of chlorophenols from wastewaters by advanced oxidation processes. Sep. Purif. Rev. 2013;42(4):263–295. [Google Scholar]
- 152.Eng Y.Y., Sharma V.K., Ray A.K. Photocatalytic degradation of nonionic surfactant, Brij 35 in aqueous TiO2 suspensions. Chemosphere. 2010;79(2):205–209. doi: 10.1016/j.chemosphere.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 153.da Silva S.W., Klauck C.R., Siqueira M.A., Bernardes A.M. Degradation of the commercial surfactant nonylphenol ethoxylate by advanced oxidation processes. J. Hazard. Mater. 2015;282:241–248. doi: 10.1016/j.jhazmat.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 154.Bokare A.D., Choi W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014;275:121–135. doi: 10.1016/j.jhazmat.2014.04.054. [DOI] [PubMed] [Google Scholar]
- 155.Chitra S., Paramasivan K., Shanmugamani A., Rao S., Paul B. Advanced oxidation processes for the treatment of surfactant wastes. J. Civ. Eng. Archit. Res. 2014;1(3):163–173. [Google Scholar]
- 156.Mondal B., Adak A., Datta P. Uv-h2o2 advanced oxidation of anionic surfactant: reaction kinetics, effects of interfering substances and operating conditions. Environ. Eng. Manag. J. 2019;18(6):1245–1254. [Google Scholar]
- 157.Abdullah A., Quinete N.S., Gardinali P., O’Shea K. Investigation of ultrasonically induced degradation of tris (2-chloroethyl) phosphate in water. J. Environ. Eng. 2020;146(10):04020117. [Google Scholar]
- 158.Makino K., Mossoba M.M., Riesz P. Chemical effects of ultrasound on aqueous solutions. Evidence for hydroxyl and hydrogen free radicals (. cntdot. OH and. cntdot. H) by spin trapping. J. Am. Chem. Soc. 1982;104(12):3537–3539. [Google Scholar]
- 159.Eren Z., O’Shea K. Hydroxyl radical generation and partitioning in degradation of methylene blue and DEET by dual-frequency ultrasonic irradiation. J. Environ. Eng. 2019;145(10):04019070. [Google Scholar]
- 160.Kıdak R., Doğan Ş. Medium-high frequency ultrasound and ozone based advanced oxidation for amoxicillin removal in water. Ultrason. Sonochem. 2018;40:131–139. doi: 10.1016/j.ultsonch.2017.01.033. [DOI] [PubMed] [Google Scholar]
- 161.Mungray A.K., Kumar P. Anionic surfactants in treated sewage and sludges: risk assessment to aquatic and terrestrial environments. Bioresour. Technol. 2008;99(8):2919–2929. doi: 10.1016/j.biortech.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 162.González S., Petrovic M., Barceló D. Removal of a broad range of surfactants from municipal wastewater–comparison between membrane bioreactor and conventional activated sludge treatment. Chemosphere. 2007;67(2):335–343. doi: 10.1016/j.chemosphere.2006.09.056. [DOI] [PubMed] [Google Scholar]
- 163.Van Ginkel C. Complete degradation of xenobiotic surfactants by consortia of aerobic microorganisms. Biodegradation. 1996;7(2):151–164. doi: 10.1007/BF00114627. [DOI] [PubMed] [Google Scholar]
- 164.Palmer M., Hatley H. The role of surfactants in wastewater treatment: impact, removal and future techniques: a critical review. Water Res. 2018;147:60–72. doi: 10.1016/j.watres.2018.09.039. [DOI] [PubMed] [Google Scholar]
- 165.Haggensen F., Mogensen A.S., Angelidaki I., Ahring B.K. Anaerobic treatment of sludge: focusing on reduction of LAS concentration in sludge. Water Sci. Technol. 2002;46(10):159–165. [PubMed] [Google Scholar]
- 166.Mogensen A.S., Ahring B.K. Formation of metabolites during biodegradation of linear alkylbenzene sulfonate in an upflow anaerobic sludge bed reactor under thermophilic conditions. Biotechnol. Bioeng. 2002;77(5):483–488. doi: 10.1002/bit.10195. [DOI] [PubMed] [Google Scholar]
- 167.Chelliapan S., Wilby T., Sallis P.J. Performance of an up-flow anaerobic stage reactor (UASR) in the treatment of pharmaceutical wastewater containing macrolide antibiotics. Water Res. 2006;40(3):507–516. doi: 10.1016/j.watres.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 168.Oktem Y.A., Ince O., Sallis P., Donnelly T., Ince B.K. Anaerobic treatment of a chemical synthesis-based pharmaceutical wastewater in a hybrid upflow anaerobic sludge blanket reactor. Bioresour. Technol. 2008;99(5):1089–1096. doi: 10.1016/j.biortech.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 169.Aloui F., Kchaou S., Sayadi S. Physicochemical treatments of anionic surfactants wastewater: effect on aerobic biodegradability. J. Hazard. Mater. 2009;164(1):353–359. doi: 10.1016/j.jhazmat.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 170.Kim S., Seo A.Y., Lee T.G. Functionalized cellulose to remove surfactants from cosmetic products in wastewater. Carbohydr. Polym. 2020;236:116010. doi: 10.1016/j.carbpol.2020.116010. [DOI] [PubMed] [Google Scholar]
- 171.Kaleta J., Elektorowicz M. The removal of anionic surfactants from water in coagulation process. Environ. Technol. 2013;34(8):999–1005. doi: 10.1080/09593330.2012.733415. [DOI] [PubMed] [Google Scholar]
- 172.Khorsandi H., Mohammadi A., Kariminejad F., Haghighi M., Karimzadeh S., Khorsandi J., Aghapour A. Optimizing linear alkyl benzene sulfonate removal using Fenton oxidation process in Taguchi method. J. Water Chem. Technol. 2016;38(5):266–272. [Google Scholar]
- 173.Mousavi S., Mahvi A., Nasseri S., Ghaffari S. Effect of Fenton process (H2O2/FE2+) on removal of linear alkylbenzene sulfonate (LAS) using centeral composite design and response surface methodology. J. Environ. Health Sci. Eng. 2011;8(2):111–116. [Google Scholar]
- 174.Valizadeh S., Younesi H., Bahramifar N. Highly mesoporous K2CO3 and KOH/activated carbon for SDBS removal from water samples: batch and fixed-bed column adsorption process. Environ. Nanotechnol. Monit. Manag. 2016;6:1–13. [Google Scholar]
- 175.Zhang R., Somasundaran P. Advances in adsorption of surfactants and their mixtures at solid/solution interfaces. Adv. Colloid Interf. Sci. 2006;123:213–229. doi: 10.1016/j.cis.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 176.Deng Y., Zhao R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015;1(3):167–176. [Google Scholar]
- 177.Stasinakis A. Use of selected advanced oxidation processes (AOPs) for wastewater treatment–a mini review. Glob. NEST J. 2008;10(3):376–385. [Google Scholar]
- 178.Oller I., Malato S., Sánchez-Pérez J. Combination of advanced oxidation processes and biological treatments for wastewater decontamination—a review. Sci. Total Environ. 2011;409(20):4141–4166. doi: 10.1016/j.scitotenv.2010.08.061. [DOI] [PubMed] [Google Scholar]
- 179.Li F., Wichmann K., Otterpohl R. Review of the technological approaches for grey water treatment and reuses. Sci. Total Environ. 2009;407(11):3439–3449. doi: 10.1016/j.scitotenv.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 180.Aboulhassan M., Souabi S., Yaacoubi A., Baudu M. Removal of surfactant from industrial wastewaters by coagulation flocculation process. Int. J. Environ. Sci. Technol. 2006;3(4):327–332. [Google Scholar]
- 181.Gönder Z., Vergili I., Kaya Y., Barlas H. Adsorption of cationic and anionic surfactants onto organic polymer resin Lewatit VPOC 1064 MD PH. Environ. Geochem. Health. 2010;32(4):267–273. doi: 10.1007/s10653-010-9297-7. [DOI] [PubMed] [Google Scholar]
- 182.Harutyunyan L., Pirumyan G. Purification of waters from anionic and cationic surfactants by natural zeolites. ԵՊՀ Գիտական տեղեկագիր-քիմիա և կենսաբանություն. 2015;236(1):21–28. [Google Scholar]
- 183.Gurses A., Yalcin M., Sozbilir M., Dogar C. The investigation of adsorption thermodynamics and mechanism of a cationic surfactant, CTAB, onto powdered active carbon. Fuel Process. Technol. 2003;81(1):57–66. [Google Scholar]
- 184.Zhang C., Wen H., Huang Y., Shi W. Adsorption of anionic surfactants from aqueous solution by high content of primary amino crosslinked chitosan microspheres. Int. J. Biol. Macromol. 2017;97:635–641. doi: 10.1016/j.ijbiomac.2017.01.088. [DOI] [PubMed] [Google Scholar]
- 185.Pal A., Pan S., Saha S. Synergistically improved adsorption of anionic surfactant and crystal violet on chitosan hydrogel beads. Chem. Eng. J. 2013;217:426–434. [Google Scholar]
- 186.Delhiraja K., Vellingiri K., Boukhvalov D.W., Philip L. Development of highly water stable graphene oxide-based composites for the removal of pharmaceuticals and personal care products. Ind. Eng. Chem. Res. 2019;58(8):2899–2913. [Google Scholar]
- 187.Jun B.-M., Kim S., Heo J., Her N., Jang M., Park C.M., Yoon Y. Enhanced sonocatalytic degradation of carbamazepine and salicylic acid using a metal-organic framework. Ultrason. Sonochem. 2019;56:174–182. doi: 10.1016/j.ultsonch.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 188.Balarak D., Mostafapour F.K., Akbari H., Joghtaei A. Adsorption of amoxicillin antibiotic from pharmaceutical wastewater by activated carbon prepared from Azolla filiculoides. J. Pharm. Res. Int. 2017:1–13. [Google Scholar]
- 189.Elmolla E.S., Chaudhuri M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination. 2010;252(1–3):46–52. doi: 10.1016/j.jhazmat.2009.08.104. [DOI] [PubMed] [Google Scholar]
- 190.Ahmed M.M., Chiron S. Solar photo-Fenton like using persulphate for carbamazepine removal from domestic wastewater. Water Res. 2014;48:229–236. doi: 10.1016/j.watres.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 191.Motteran F., Braga J.K., Sakamoto I.K., Varesche M.B.A. Methanogenic potential of an anaerobic sludge in the presence of anionic and nonionic surfactants. Int. Biodeterior. Biodegradation. 2014;96:198–204. [Google Scholar]
- 192.Nasseri S., Yavarian J., Baghani A.N., Azad T.M., Nejati A., Nabizadeh R., Hadi M., Jandaghi N.Z.S., Vakili B., Vaghefi S.K.A. The presence of SARS-CoV-2 in raw and treated wastewater in 3 cities of Iran: Tehran, Qom and Anzali during coronavirus disease 2019 (COVID-19) outbreak. J. Environ. Health Sci. Eng. 2021:1–12. doi: 10.1007/s40201-021-00629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]