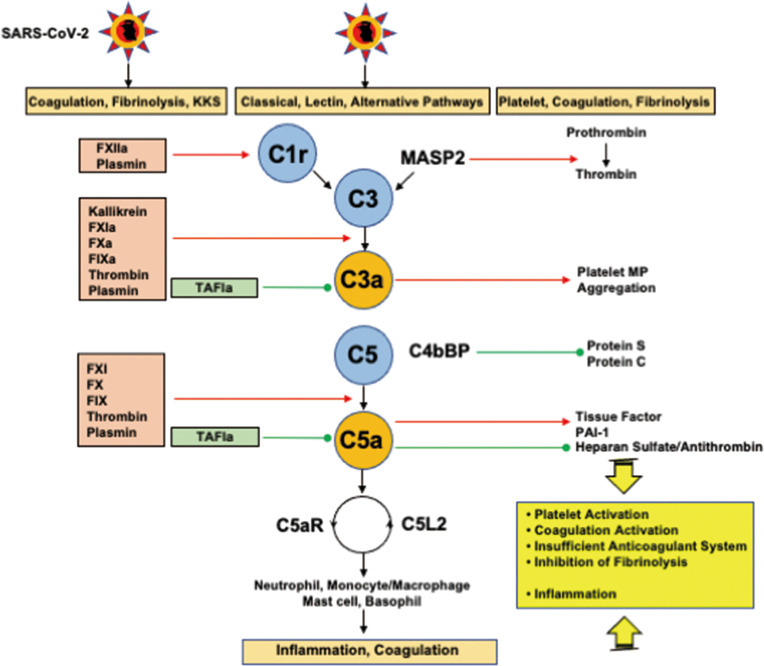
Figure 4.
Interplays among coagulation, fibrinolysis, complement pathways, and inflammation. FXIIa and plasmin activate C1r to initiate the classical pathway. In addition to kallikrein, FXIa, FXa, FIXa, thrombin, and plasmin cleave C3 and C5 to generate C3a and C5a. Importantly, kallikrein, thrombin, and plasmin are able to directly activate C3, and thrombin can generate C5a without the participation of C3. TAFIa is the only molecule that blocks C3a and C5a. MASP2 and MAC (not shown in the figure) cleave prothrombin into thrombin. C3a generates platelet procoagulant microparticles and induce platelet aggregation. C5a expresses tissue factor and PAI-1 on neutrophils and mast cells, respectively through C5aR, and inhibits the antithrombin-mediated anticoagulation pathway through the shedding of glycocalyx heparan sulfate. Complex formation between C4bBP and protein S impairs the functional property of protein S, a cofactor of protein C, resulting in a decrease in the conversion of protein C to activated protein C. C5a finally induces inflammation via inflammatory cells through C5aR and C5L2. These mutual actions of the serine protease network contribute to inflammation and thrombus formation at the site of infection, if the infection is sufficiently severe, disseminating to the systemic circulation, and DIC associated with SIRS ensues. C5aR, C5a receptor; C5L2, C5-like receptor2; C4bBP, C4b binding protein; DIC, disseminated intravascular coagulation; MAC, membrane attack complex; MASP, mannose-binding lectin-associated serine protease 2; PAI-1, plasminogen activator inhibitor-1; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SIRS, systemic inflammatory response syndrome; TAFIa, activated thrombin-activatable fibrinolysis inhibitor. Red arrows indicate activation and green lines with circle-head indicate inhibition.