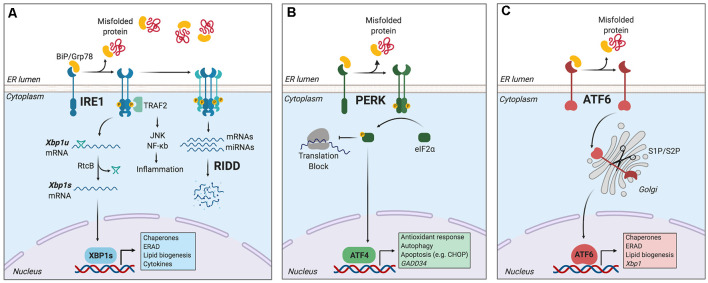
Figure 1.
The unfolded protein response (UPR). Endoplasmic reticulum (ER) stress induces an adaptive response known as the unfolded protein response (UPR), which is controlled by three main ER-resident sensors: IRE1, PERK, and activating transcription factor-6 (ATF6). (A) IRE1 is activated by oligomerization and trans-phosphorylation upon binding of unfolded proteins and release of the chaperone BiP. IRE1 autophosphorylation leads to the activation of its RNase domain and the processing of the mRNA encoding for X-box binding protein 1 (XBP1s), a transcriptional factor that upregulates genes involved in protein folding and quality control, in addition to regulating ER/Golgi biogenesis and ER-mediated degradation (ERAD), lipid biogenesis and cytokine production. Additionally, IRE1 RNase also degrades a subset of specific RNAs and microRNAs, a process termed Regulated IRE1-Dependent Decay (RIDD). (B) Upon activation, PERK phosphorylates the eukaryotic initiation factor-2α (eIF2α), decreasing the synthesis of proteins and the overload of misfolded proteins at the ER. PERK phosphorylation also leads to the specific translation of ATF4, a transcription factor that promotes the expression of genes related to amino acid metabolism, antioxidant response, autophagy, and apoptosis. (C) ATF6 is activated upon release of BiP and is translocated to the Golgi, where it undergoes sequential cleavage and removal of its luminal domain. The remaining transactivation domain of ATF6 moves to the nucleus and coordinates the expression of genes encoding ER chaperones, ER-associated protein degradation (ERAD) components, and molecules involved in lipid biogenesis. Figure created with BioRender.com.