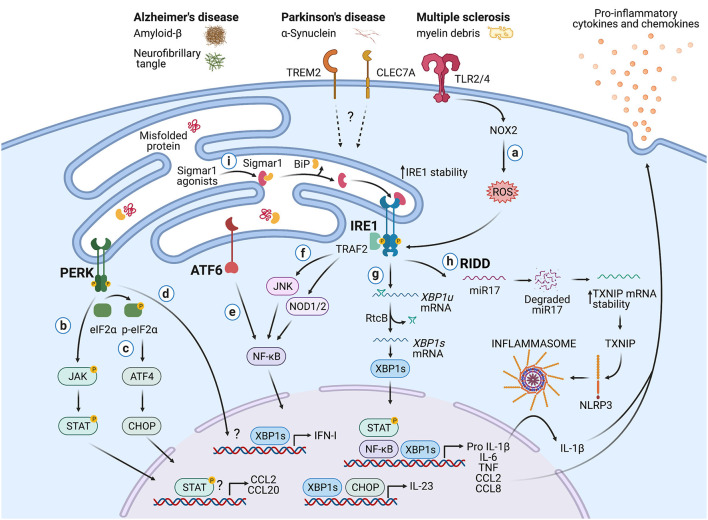
Figure 2.
Potential roles of the UPR in immune cells during neurodegeneration. Protein aggregates and myelin debris can promote inflammation via triggering of innate receptors and activation of the UPR, which in turn could increase inflammation in neurodegenerative diseases mainly by enhancing the production of proinflammatory cytokines and chemokines. (a) Detection of proteins aggregates (Amyloid-β, α-synuclein, neurofibrillary tangles) and myelin debris through TLR2 and TLR4 (and probably others pattern recognition receptors) present on immune cells can activate the IRE1/XBP1s axis through reactive oxygen species (ROS) production by NOX2. (b) PERK can modulate the production of the pro-inflammatory cytokines IL-6, TNF, IL-1β, and the chemokines CCL2 and CCL20 through activation of JAK1-STAT3 and JAK2-STAT1 signaling pathways. (c) PERK also can control the synthesis of IL-23 via CHOP. (d) Additionally, PERK could control the synthesis of type I interferons. (e) ATF6 via NF-κB can enhance the production of the cytokines IL-6, TNF, and the chemokines CCL2 and CCL8. (f) The kinase domain of IRE1 can modulate the production of IL-6, TNF, and IL-1β through activation of NF-κB via JNK and NOD1/2 receptors. (g) BP1s can promote optimal production of IL-6, TNF, and type I IFNs and also favors the synthesis of IL-23. (h) IRE1 RNase via RIDD activates the NLRP3 inflammasome through degradation of the TXNIP-destabilizing microRNA miR-17, leading to IL-1β production. (i) Sigmar1 forms a complex with binding immunoglobulin protein (BiP) under normal conditions, but Sigmar1 agonists can dissociate Sigmar1 from Bip to induce its action as a chaperone protein. Sigmar1 interacts with IRE1 and stabilizes it, prolonging IRE1/XBP1s signaling. Figure created with BioRender.com.