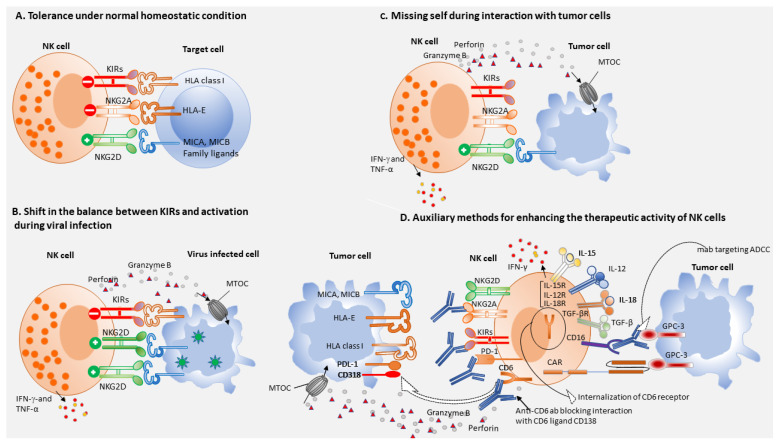
Figure 1.
Shift in the balance between inhibitory and activation signaling; auxiliary methods for enhancing the therapeutic efficacy of NK cell-based immunotherapy. NK cells express a broad spectrum of receptors with activating or inhibitory functions and the balance between signaling inputs through these receptors shifts the equilibrium towards tolerance or activation of cytotoxicity against the target cells. (A). Under normal homeostatic condition, when the overall inhibitory signaling strength outweighs activating receptor signaling, NK cell activation is aborted resulting in tolerance. (B). During viral infection, cells upregulate stimulatory ligands for NK cell activating receptor NKG2D and as a result activation signal surpasses signaling through the inhibitory receptors KIRS and NKG2A, resulting in cytokine release and cytolysis of target cells. (C). Class I MHC ligands of NK cell inhibitory receptors are downregulated in tumor cells during malignant transformation and the loss of inhibitory signals causes positive signaling which leads to NK cell activation and tumor cell killing, referred to the “missing-self” phenomena. (D). Immune-checkpoint inhibitors could potentially relieve suppression of NK cell-mediated cytotoxicity by preventing inhibitory signaling through PD-1, CD6, NKG2A, and killer immunoglobulin-like receptors (KIRs). In addition, activation of members of the natural cytotoxicity receptor family, such as NKG2D, results in the release of preformed cytolytic granules containing granzyme B and perforin. Pro-inflammatory cytokines such as IL-12 and IL-18 enhance NK cell effector function and cytokine secretion, whereas anti-inflammatory cytokine TGFβ inhibits NK cell function. IL-15 is a crucial homeostatic cytokine for NK cells and exogenous IL-15 can cause NK cell activation. NK cells expressing Fc receptors for antibodies, can recognize and kill sensitized tumor cells through ADCC. Genetically modified CAR NK cells accelerate the antitumor activity of adoptive NK cell therapies.