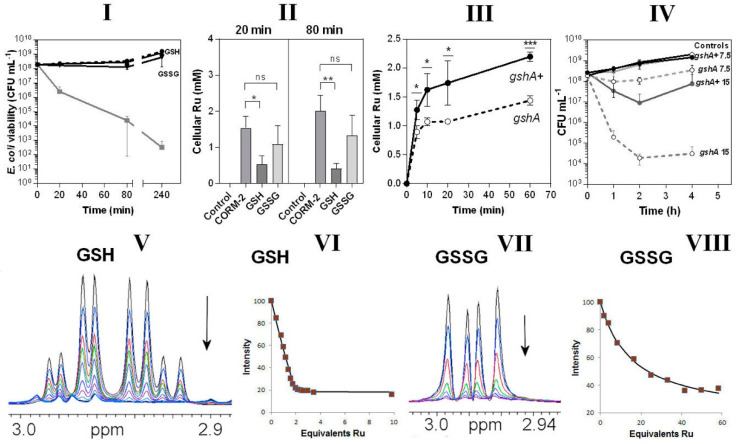
Figure 6.
Glutathione is an important intracellular and extracellular target of CORM-2 (I–IV) and CORM-2 has a much higher affinity for reduced glutathione (GSH) than oxidised glutathione (GSSG) as investigated by 1H NMR (V–VIII). In I, the effects are shown of extracellular reduced glutathione (GSH) or oxidised glutathione (GSSG). The grey line shows cell viability with CORM-2 alone. GSH fully restored viability to that of the no-CORM control (dashed line). II shows CORM-induced intracellular Ru accumulation in the absence or presence of GSH or GSSG (* p ≤ 0.05, ** p ≤ 0.01, assessed via unpaired t-test). CORM-2 stocks were incubated with a 2-fold excess of the compound prior to addition of 30 µM CORM-2. (III) Uptake of CORM-2-derived Ru is significantly reduced in a glutathione-deficient mutant (gshA, open symbols, dashed line) compared to the GshA+ wild-type parent strain (gshA+, solid line) (* p ≤ 0.05, *** p ≤ 0.001, assessed via unpaired t-test). (IV) Culture viability (CFU mL−1) of the glutathione-deficient strain compared to the parent strain following addition of 7.5 or 15 µM CORM-2. Data in I–IV represent 3 biological repeats ± SD. (V) NMR spectra of the Cys Hβ resonances of GSH (1 mM) on titration with 0–5 mM CORM-2 and (VI) corresponding binding curve. (VII) NMR spectra of the Cys Hβ resonances of GSSG (0.5 mM) on titration with 0–14.5 mM CORM-2 and (VIII) corresponding binding curve. In each titration the arrow shows the direction of increasing [CORM-2]. The estimated Kd of CORM-2 to GSH was determined to be: 2 ± 1 μM for GSH (VI) and 25 ± 5 mM for GSSG (VIII). Titrations were performed in 30 mM KPi buffer pH 7.4 and pH was maintained by adjustment with KOH throughout the experiment.