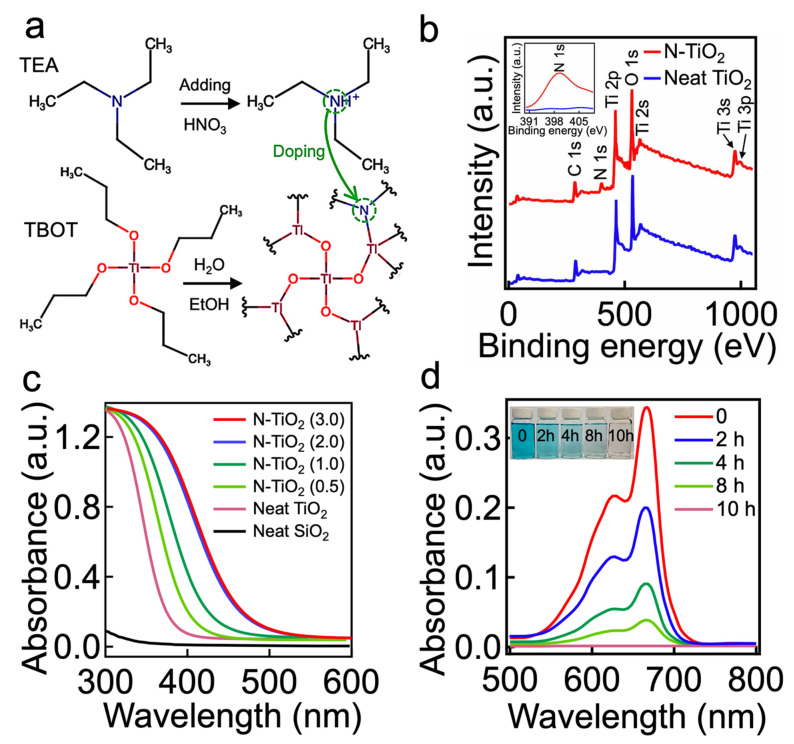
Figure 1.
(a) Schematic illustrating the synthesis of N-TiO2 nanoparticles by utilizing titanium butoxide (TBOT) and triethylamine (TEA) as a TiO2 precursor and nitrogen dopant, respectively. (b) The XPS spectrum of N-TiO2, exhibiting characteristic peaks of N 1s, Ti 2p, and O 1s. The spectrum of a neat TiO2 is also shown for comparison. The inset shows the core level spectrum of characteristic N 1s. (c) Ultraviolet–visible (UV-Vis) absorption spectra of N-TiO2 synthesized by varied molar ratios of TEA to TBOT (e.g., 0.5, 1.0, 2.0, and 3.0). Neat TiO2 and SiO2 absorption spectra are also shown for comparison. (d) UV-Vis absorption spectra of water dissolved with N-TiO2 and Solvent Blue dye as a function of the visible light irradiation time. The inset is a photograph showing the water dissolved with Solvent Blue dye after visible light irradiation for 2 h, 4 h, 8 h, and 10 h. The as-prepared water dissolved with Solvent Blue dye (concentration = 0.5 wt%) is also shown.