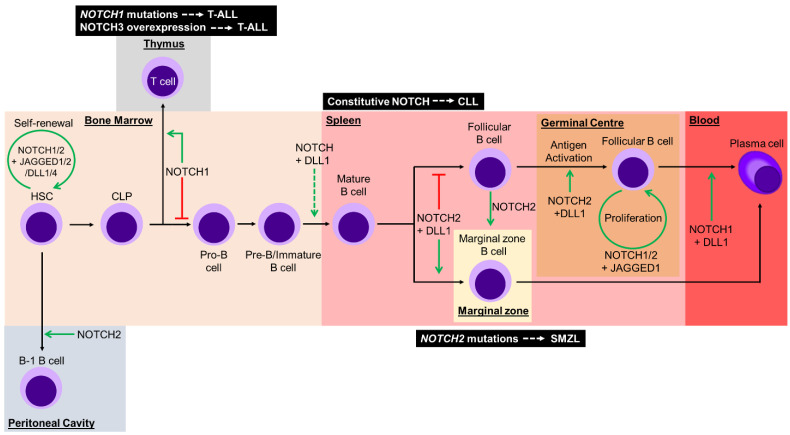
Figure 3.
Development of hematopoietic stem cells (HSCs) from common lymphoid progenitors (CLPs) through to terminally differentiated plasma cells requires mediation of Notch signals at multiple stages. Within the bone marrow, HSCs are maintained in their pluripotent state through Notch signaling with surrounding stromal cells. NOTCH2 signaling promotes peritoneal B1 B cell differentiation. CLPs encounter a Notch signaling checkpoint that determines T or B cell fate through activation or inhibition of NOTCH1 signaling, respectively. Notch signaling with DELTA-LIKE1 (DLL1) is proposed to function as another checkpoint alongside negative selection against self-antigen of pre-B/immature B cells, prior to migration from the bone marrow into secondary lymphoid organs. Within the spleen, mature B cells develop into marginal zone B cells with NOTCH2 and DLL1 mediating Notch activation, whilst the absence of Notch signaling regulates follicular B cells. Notch signal is capable of mediating plasticity and inducing transdifferentiation from follicular B cells to functional marginal zone B cells. Upon antigen encounter in the germinal center, NOTCH2- and DLL1-mediated Notch signaling induces follicular B cell activation. Proliferation of activated follicular B cells is mediated by Notch activation through JAGGED1. Lastly, NOTCH1 and DLL1 mediated Notch signaling promotes terminal differentiation to plasma cells. Green arrows represent Notch signaling, whilst the red lines represent the absence of Notch signaling. The green dashed arrow represents a yet to be elucidated requirement for Notch signaling. Dysregulation of Notch signaling can contribute to lymphoid malignancies (black boxes) at different stages of development. T-ALL: T-acute lymphoblastic leukemia; CLL: chronic lymphocytic leukemia; SMZL: splenic marginal-zone lymphoma.