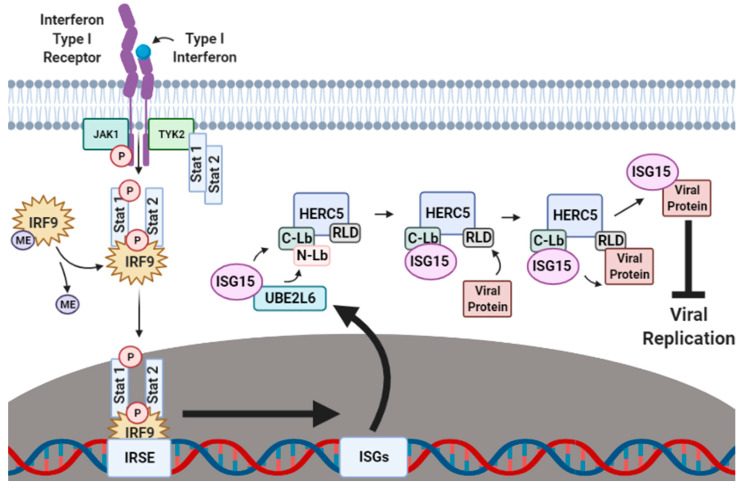
Figure 1.
Interferon-induced ISGylation of viral proteins. During the initial stages of infection, cellular IFN-α/β surface receptors are bound by IFN-α/β-specific ligands that expose receptor phosphorylation sites to the cytoplasm. Janus kinase 1 (JAK1) and tyrosine-protein kinase 2 (TYK2) phosphorylate exposed cytoplasmic IFN-α/β receptor sites, thus recruiting the nuclear transcriptional regulators signal transducer and activator of transcription proteins 1 and 2 (STAT1 and STAT2) to also be phosphorylated by TYK2. In the cytoplasm, phosphorylated STAT1 and STAT2 form a ternary complex with methylated IRF9 which is then demethylated to signal for the migration of the STAT1–STAT2–IRF9 (SSI) complex to the nucleus. Upon nuclear entry, the SSI complex binds to the ISG promoter region interferon-stimulated response element (ISRE) to upregulate the transcription of several hundred ISGs, including ISG15, UBE1L, UBE2L6 and HERC5. Translated UBE1L, UBE2L6 and HERC5 proteins are subsequently targeted to the perinuclear regions of the cytoplasm where they work in tandem to charge, transfer and attach ISG15 onto respective viral protein substrates to inhibit viral stability, transport and reassembly. ME, methylation. This figure was created with Biorender™.