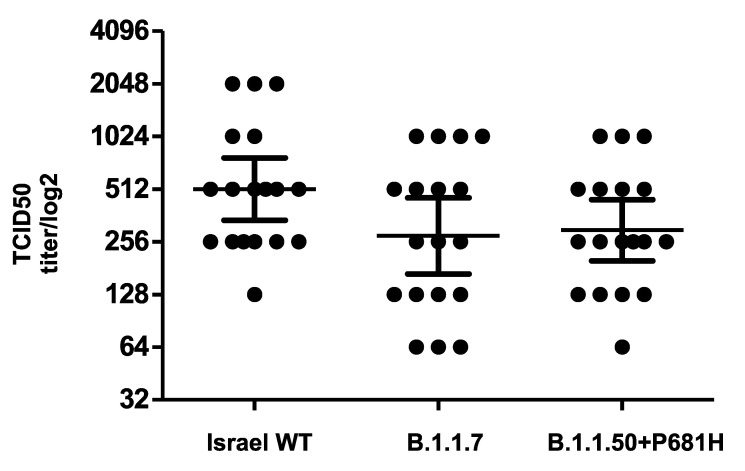
Figure 3.
Neutralization of the B.1.1.50 + P681H variant. Neutralization assays were carried out with VERO-E6 cells infected with the B.1.1.50 + P681H variant, B.1.1.7 variant and an Israel WT strain from Israel, using sera from fully vaccinated individuals. On day 6, plates were colorized overnight with Gentian violet +4% formaldehyde solution for virus neutralization. Titers were calculated by qualitative measurements of the cytopathic effect for each patient. Bars represent the geometric mean titer (GMT) and 95% confidence intervals.