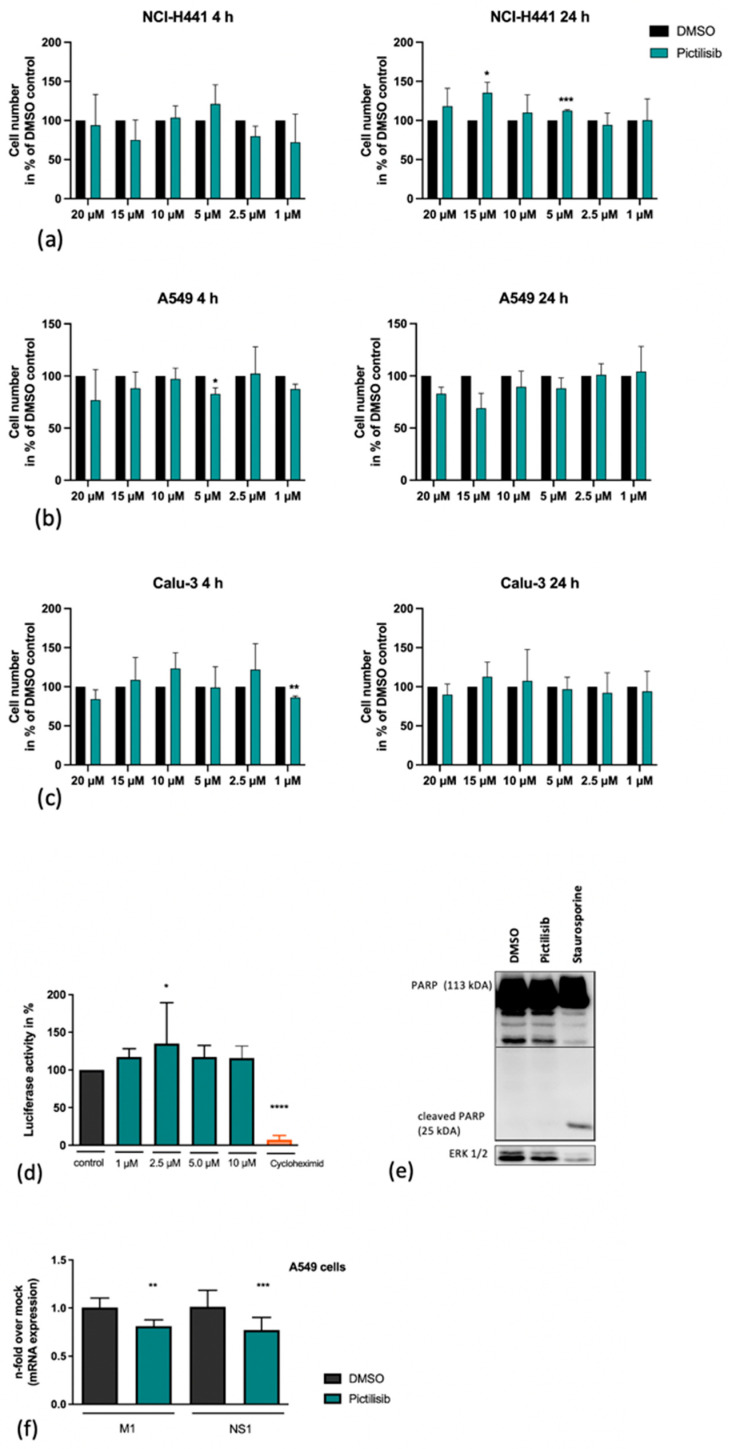
Figure 3.
Pictilisib treatment does not affect cell viability. NCl-H441 (a), A549 cells (b) and Calu-3 cells (c) were treated with increasing amounts of Pictilisib (1–20 µM) or DMSO in 500 µL of medium with 10% FCS. After the indicated times cells were washed with PBS and detatched with 150 µL/well Trypsin/EDTA. Subsequently, the procedure was stopped by adding 350 µL/well medium with 10% FCS. 20 µL of suspension was supplemented with 20 µL 0.1% Trypan Blue and used for counting. (d) A549 cell were transfected with a constitutive active CMV promoter luciferase plasmid. Eight hours post transfection cells were left untreated or treated with the indicated amounts of Pictilisib or cycloheximide (100 µg mL−1) respectively for 18 h. (d–f) Staurosporine (1 µM) or cycloheximide (100 µg mL−1)-treated cells were used as positive controls. The cleavage of PARP (113 kDa) by Pictilisib was proven by Western blot analysis (e). For this, cells were lysed on ice with RIPA buffer and transferred to SDS-PAGE and afterwards blotted onto a nitrocellulose membrane. The cleaved PARP was visualized by use of a PARP antibody (e). A549 cells (f) were left untreated or pre-incubated with Pictilisib (10 µM) or DMSO for 30 min prior to infection with influenza PR8M (H1N1) (MOI 5). After 30 min of infection, viral inoculum was removed, and cells were supplemented with medium containing Pictilisib or DMSO for 8 h. The mRNA expression of M1 and NS1 was blocked significantly by Pictilisib (f). Data represent means + SD of three independent experiments including four biological samples. DMSO-treated cells were arbitrarily set as 100%. Statistical analysis was performed by one-way ANOVA followed by multiple comparisons test. (**** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05).