Abstract
Objectives
Evaluation of remdesivir, an RNA polymerase inhibitor, for effectiveness in adults with COVID-19.
Data sources
Electronic search for eligible articles of PubMed, Cochrane Central and clinicaltrials.gov was performed on 20 September 2020.
Participants and study eligibility criteria
Only randomised controlled trials (RCTs) evaluating efficacy of remdesivir in COVID-19 were included for meta-analysis.
Interventions
Remdesivir was compared with standard of care.
Primary and secondary outcomes
Primary outcome was mortality and secondary outcomes were time to clinical improvement and safety outcomes like serious adverse events, respiratory failure.
Study appraisal and synthesis methods
Data synthesis was done with Cochrane review manager 5 (RevMan) V.5.3. Cochrane risk of bias V.2.0 tool was used for methodological quality assessment. The GRADE pro GDT was applied for overall quality of evidence.
Results
52 RCTs were screened and 4 studies were included in analysis, with total of 7324 patients. No mortality benefit was observed with remdesivir versus control group (OR=0.92 (95% CI 0.79 to 1.07), p=0.30, moderate quality evidence). Significantly higher rates of clinical improvement (OR=1.52 (95% CI 1.24 to 1.87), p<0.0001, low quality) and faster time to clinical improvement (HR=1.28 (95% CI 1.12 to 1.46), p=0.0002, very low quality) was observed with remdesivir versus control group. Significant decrease was found in the risk of serious adverse events (RR=0.75 (95% CI 0.62 to 0.90), p=0.0003, low quality); however, no difference was found in the risk of respiratory failure (RR=0.85 (95% CI 0.41 to 1.77), p=0.67, very low quality evidence) with remdesivir.
Conclusions
As per the evidence from current review, remdesivir has shown no mortality benefit (moderate quality evidence) in the treatment of COVID-19. From a cost–benefit perspective, it is our personal opinion that it should not be recommended for use, especially in low and lower middle income countries.
Trial registration number
PROSPERO registration number: CRD42020189517.
Keywords: COVID-19, immunology, clinical pharmacology, respiratory infections
Strengths and limitations of this study.
Four randomised controlled trials (RCTs) were included in our analysis with total sample size of 7324 patients.
Risk of bias (ROB) of RCTs was done using Cochrane ROB-2 scale.
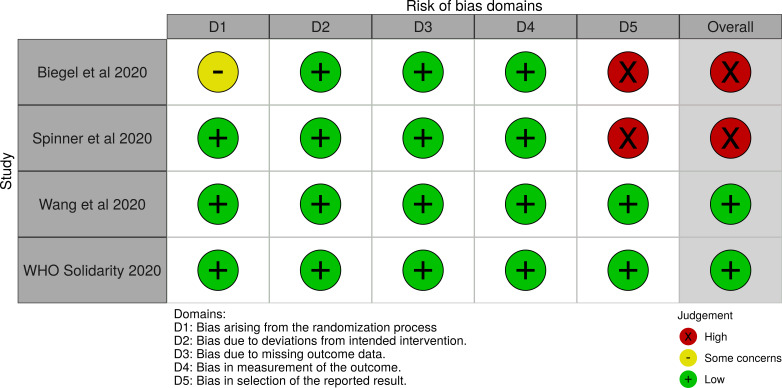
ROB-2 showed low ROB for WHO Solidarity trial and Wang et al and high ROB for Beigel et al and Spinner et al
GRADE was applied and overall evidence suggested no mortality benefit with remdesivir (moderate quality evidence).
Cost–benefit analysis revealed higher cost with no mortality benefit.
Introduction
COVID-19 has created a pandemic all over the world.1 2 The global pandemic of SARS-CoV-2 infections has affected more than 141 million people worldwide and has been the cause of 3.026 million deaths globally by 18 April 2021 as per COVID-19 statistics data. Around 120 million people have recovered and as the trend suggests most of them stay asymptomatic and few of them develop pneumonia-like symptoms that does not require oxygen support.3 A very small percentage get critical to the limit of hypoxia, acute respiratory distress syndrome and multiorgan failure. Among these critical patients who are being put on mechanical ventilation, half of them die.
The search for an effective therapy or preventive modality has become the utmost need of the hour. There are few proposed and approved drugs with some antiviral action and they are under investigation simultaneously across the globe. But as yet no proven effective therapy for SARS-CoV-2 has been accepted widely. Among the few promising therapies available remdesivir, a viral RNA polymerase inhibitor has been recommended by US Food and Drug Administration (FDA) as a drug for compassionate use for treatment of patients with COVID-19. Remdesivir, a nucleoside analogue prodrug, has shown inhibitory effects on SARS-CoV-2, both in vitro and in animal models. However, even for the above-mentioned studies, contrasting results have been reported in different nations like China and USA.3 Varied study designs,4 5 genetic reasons and different treatment regimens (5 or 10 days) have been attributed for this difference.
Only two randomised controlled trials (RCT) have shown efficacy of remdesivir in patients with COVID-19. Many RCTs are undergoing to assess the benefit–risk ratio of remdesivir. Current review was planned to assess the mortality and clinical benefit in addition to safety of remdesivir in the treatment of COVID-19 caused by SARS-CoV-2.
Methods
Protocol and registration
Review was done following the “PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses”) statement. “PROSPERO (International Prospective Register of Systematic Reviews) database” registration was done with study number as CRD42020189517.
Inclusion criteria
Exclusively RCTs evaluating role of remdesivir compared with standard care in COVID-19 were included. Observational studies, review articles, case reports or case series were excluded.
Search and selection of studies
Electronic literature search was performed in PubMed, Cochrane Central Register of Controlled Trials, in addition to clinicaltrials.gov on 20 September 2020, to identify the relevant published articles. Additional search was done in November 2020 for results of completed trials. Bibliographic search of published articles were also done manually to identify more studies. Only English language studies published were included. Search was performed using medical headings like ‘SARS-CoV-2’, ‘COVID-19’, ‘Remdesivir’, ‘COVID-19’, ‘novel coronavirus’. RCT restriction was applied. PubMed search strategy is given in online supplemental file 1).
bmjopen-2020-048416supp003.pdf (101.8KB, pdf)
After removal of duplicate articles, two independent authors reviewed the studies for inclusion in review.
Data extraction
Study design, remdesivir doses and regimens, total subjects along with their characteristics, efficacy and safety outcomes were extracted and filled on a prestructured form.
Study objectives
The primary objective of review was assessment of mortality (defined as deaths in each group). The secondary outcomes were clinical improvement and virological cure. In addition, serious adverse events (AEs) and other safety parameters were assessed. Cost–benefit analysis was also performed for remdesivir.
Quality assessment
Two authors independently (DK and AC) performed risk of bias (ROB) of RCTs using Cochrane Collaboration ROB-2.6 Overall assessment was recorded as high, low and some concerns. Synthesis of ROB plots was done using online software Robvis (visualisation tool).7
For publication bias assessment, funnel plot asymmetry was not assessed as studies were less than five. However, Egger’s regression test was applied.
Data synthesis and summary measures
Mortality and other outcome data were presented as OR or HR with 95% CIs. Synthesis of data was done using “Review Manager 5 (RevMan) Version 5.3” (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).8 The heterogeneity among RCTs included in review was judged with I2.9 10 The results of both fixed and random effect model were assessed for interpretation.9 11
Quality of evidence—GRADE Pro GDT
GRADE pro GDT (guideline development tool) software (https://gradeproorg/.) was applied for assessment of overall quality of evidence.12 Optimal information size or sample size for either group was computed to be 1213 patients. Overall GRADE assessment was classified as high, moderate, low or very low.12
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Results
Study PRISMA flow diagram
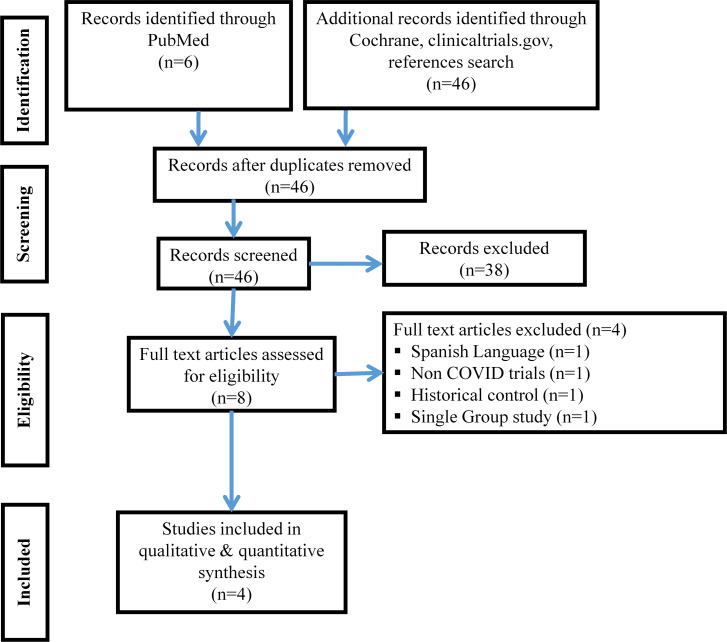
The RCTs included in review are depicted in PRISMA flow chart (figure 1). Out of total 52 records screened, 4 RCTs3 13–15 were included in analysis. One study was excluded as it was single arm study,4 one was in Spanish language and other two were non-COVID trial16 and historical control study.17
Figure 1.
PRISMA flow chart depicting study selection process.
Study characteristics
Study characteristics of RCTs of present systematic review are mentioned in table 1.
Table 1.
Characteristics of clinical studies evaluating remdesivir for treatment of COVID-19
| Author, year (study design) |
Institution/country of study conduct | Study interventions (N)/regimen | Study control (N)/regimen | Study population characteristics | Study outcomes |
|
Beigel et al 2020 (randomised controlled trial) |
Multicentre trial | Remdesivir (538); 200 mg on day 1 followed by 100 mg on days 2–10 in single daily infusions | Placebo (521) | Hospitalised adults patients with COVID-19 with evidence of lower respiratory tract involvement. |
Time to recovery: Patients in the remdesivir group had a shorter time to recovery than patients in the placebo group (median, 11 days, as compared with 15 days; rate ratio for recovery, 1.32; 95% CI (CI), 1.12 to 1.55; p<0.001 Mortality: Kaplan-Meier estimates of mortality by 14 days were 7.1% with remdesivir and 11.9% with placebo (HR for death, 0.70; 95% CI, 0.47 to 1.04) |
|
Spinner et al (randomised controlled trial) |
Multicentre trial | Remdesivir - 10 days (n=197), Remdesivir - 5 days (n=199) |
Standard care (n=200) | Confirmed SARS-CoV-2 infection and moderate COVID-19 pneumonia (pulmonary infiltrates and room-air oxygen saturation >94%) |
Day 28 Mortality rate n(%) – remdesivir 10 day=3 (2); remdesivir 5 days=2 (1), standard=4 (2) Clinical Improvement n(%) - remdesivir 10 day=174((90), remdesivir 5 day=171(90), Standard=166(83) |
|
Wang et al 2020 (randomised controlled trial) |
Department of Pulmonary and Critical Care Medicine, China-Japan Friendship Hospital, Beijing, China | Remdesivir (158); at least 1 dose after entering ICU; 200 mg on day 1 followed by 100 mg on days 2–10 in single daily infusions | Placebo (79) | Hospitalised adults patients with COVID-19 symptom onset to enrolment interval of <12 days, oxygen saturation <94% on room air or a ratio of arterial oxygen partial pressure to fractional inspired oxygen of 300 mm Hg or less, and radiologically confirmed pneumonia |
Time to clinical improvement within 28 days after randomisation: Remdesivir use was not associated with a difference in time to clinical improvement (HR 1.23 (95% CI 0.87 to 1.75)). Although not statistically significant, patients receiving remdesivir had a numerically faster time to clinical improvement than those receiving placebo among patients with symptom duration of 10 days or less (HR 1.52 (0.95 to 2.43) 28-day mortality: similar between the two groups (22(14%] died in the remdesivir group vs 10 (13%) in the placebo group; difference 1.1% (95% CI –8.1 to 10.3)). |
|
WHO Solidarity Trial 2020 (randomised controlled trial) |
WHO, Multicentric trial (405 hospitals in 30 countries) | Remdesivir (2743); day 0, 200 mg; days 1–9, 100 mg | Placebo (2708) | Hospitalised with a diagnosis of COVID-19, age ≥18 years, not known to have received any study drug, without anticipated transfer elsewhere within 72 hours |
Mortality rate: Remdesivir RR=0.95 (0.81 to 1.11, p=0.50; 301/2743 active vs 303/2708 control). Hydroxychloroquine RR=1.19 (0.89 to 1.59, p=0.23; 104/947 vs 84/906), Lopinavir RR=1.00 (0.79 to 1.25, p=0.97; 148/1399 vs 146/1372) Interferon RR=1.16 (0.96 to 1.39, p=0.11; 243/2050 vs 216/2050) |
Risk of bias
The overall ROB was judged as “Low”, as ROB for WHO solidarity trial14 and Wang et al3 was assessed as low. Study done by Beigel et al13 and Spinner et al15 was regarded as having “High” ROB. Hence, ROB assessed for outcomes having data only from Beigel et al and Spinner et al in GRADE analysis was regarded as having serious issues. The ROB of RCTs is represented in figure 2 and online supplemental figure 1.
Figure 2.
ROB-2: risk of bias in RCT evaluating remdesivir for treatment of COVID-19.
bmjopen-2020-048416supp001.pdf (854.5KB, pdf)
Efficacy outcomes
Mortality
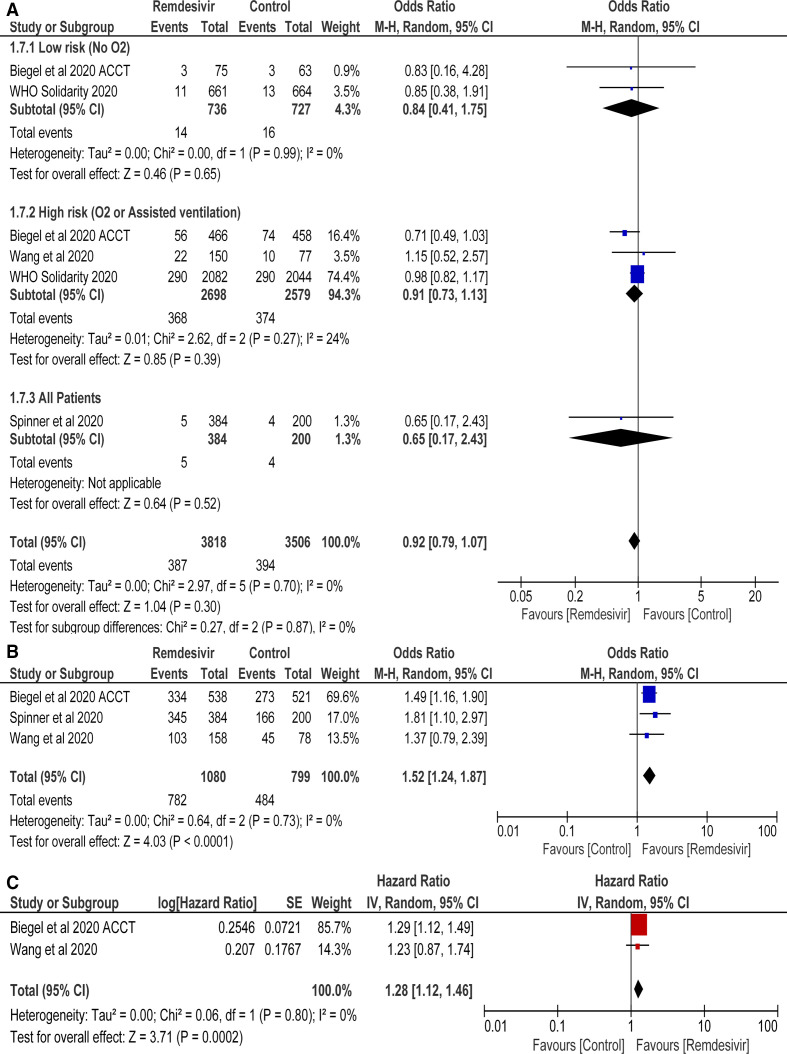
Mortality data were included from 4 RCTs with 3818 and 3506 patients in remdesivir and standard of care groups, respectively. Remdesivir was found to have no mortality benefit as compared with control group (OR=0.92 (95% CI 0.79 to 1.07), p=0.30; I2=0) (figure 3A). Subgroup analysis revealed no mortality benefit in low-risk and high-risk groups (figure 3A, online supplemental figure 2)
Figure 3.
(A) Mortality rate, (B) clinical improvement and (C) time to clinical improvement of remdesivir versus control treatment.
bmjopen-2020-048416supp002.pdf (779.8KB, pdf)
Clinical improvement
Statistically significant increase in rates of clinical improvement in remdesivir versus controls was observed (OR=1.52 (95% CI 1.24 to 1.87), p<0.0001; I2=0%) (figure 3B). Results were drawn from 3 RCTs with total of 1879 patients.
Time to clinical improvement
Pooled analysis revealed that there was significantly faster time to clinical improvement in remdesivir group as compared with controls (HR=1.28 (95% CI 1.12 to 1.46), p=0.0002; I2=0%) (figure 3C). Data extracted from 2 RCTs with total of 1292 patients.
Safety outcomes
Serious AEs
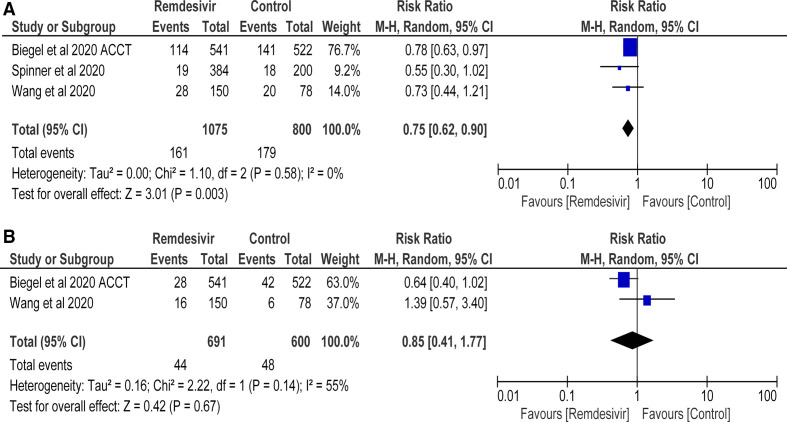
Pooled analysis revealed significant decrease in the risk of serious AEs in remdesivir group as compared with control (RR=0.75 (95% CI 0.62 to 0.90), p=0.0003; I2=0%) (figure 4A). This data were extracted from 3 RCTs with a total of 1875 patients.
Figure 4.
Number of patients with (A) serious adverse events and (B) respiratory failure (remdesivir vs control treatment).
Respiratory failure
No difference in the risk of respiratory failure between remdesivir and control groups was found (RR=0.85 (95% CI 0.41 to 1.77), p=0.67; I2=55%) (figure 4B). Findings were derived from 2 RCTs with a total of 1291 patients.
Cost–benefit analysis
The cost of remdeisvir is US$2340 per patient. There is lack of mortality benefit as per our review.
Publication bias
Though the funnel plot asymmetry was not assessed, the Egger’s regression test applied on four studies included in mortality rate assessment showed no publication bias (t = −0.5947, p=0.6123).
Grade analysis of the primary and secondary outcomes
The GRADE analysis recommendation for mortality was ‘Moderate’ evidence quality. Though there is low ROB, low heterogeneity and direct outcome but there are serious concerns with imprecision. The quality of evidence for clinical improvement and time to clinical improvement were graded as “Low” and “Very Low”, respectively. The GRADE recommendation for serious AE and respiratory failure were “Low” and “Very low” quality of evidence respectively, as there was presence of high ROB and high imprecision. The GRADE recommendation is shown in table 2.
Table 2.
GRADE recommendation for primary and secondary outcomes of use of remdesivir in COVID-19
| Certainty assessment | No of patients | Effect | Certainty Importance |
|||||||
| No of studies Study design |
Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Efficacy and safety of remdesivir | Placebo | Relative (95% CI) |
Absolute (95% CI) |
|
| Mortality at day 28 | ||||||||||
| 4 RCT |
Not serious* | Not serious | Not serious | Serious† | None | 387/3818 (10.1%) | 394/3506 (11.2%) |
OR 0.92 (0.79 to 1.07) |
8 fewer per 1000 (from 21 fewer to 7 more) |
⨁⨁⨁◯ moderate critical |
| Clinical improvement | ||||||||||
| 3 RCT |
Serious‡ | Not serious | Not serious | Serious§ | None | 782/1080 (72.4%) | 484/799 (60.6%) |
OR 1.52 (1.24 to 1.87) |
94 more per 1000 (from 50 more to 136 more) |
⨁⨁◯◯ low important |
| Time to clinical Improvement | ||||||||||
| 2 RCT |
Serious¶ | Not serious | Serious** | Serious§ | None | -/0 | -/0 |
HR 1.28 (1.12 to 1.46) |
1 fewer per 1000 (from 1 fewer to 1 fewer) |
⨁◯◯◯ very low important |
| Serious adverse events | ||||||||||
| 3 RCT |
Serious‡ | Not serious | Not serious | Serious§ | None | 161/1075 (15.0%) | 179/800 (22.4%) |
RR 0.75 (0.62 to 0.90) |
56 fewer per 1000 (from 85 fewer to 22 fewer) |
⨁⨁◯◯ low important |
| Respiratory failure | ||||||||||
| 2 RCT |
Serious¶ | Serious†† | Not serious | Serious‡‡ | None | 44/691 (6.4%) | 48/600 (8.0%) |
RR 0.85 (0.41 to 1.77) |
12 fewer per 1000 (from 47 fewer to 62 more) |
⨁◯◯◯ very low critical |
*All studies have low ROB except Biegel and Spinner et al. WHO solidarity trial contributing 77.9% wt to overall effect has low ROB. Hence overall low ROB.
†Overall information size of 1213 was achieved in either group. However, the overall effect estimate included one, hence downgraded for imprecision.
‡Biegel et al and Spinner et al have a high risk of bias (ROB) due to selective reporting of results. Hence, downgraded for ROB.
§Overall information size of 1213 was not achieved in either groups. Hence, downgraded for imprecision.
¶Biegel et al has a high risk of bias (ROB) due to selective reporting of results. Hence, downgraded for ROB.
**Time to clinical improvement is not a direct estimate of the patient’s oriented outcomes. Hence, downgraded for evidence.
††As I2 >50%, heterogeneity is significantly high. Hence, downgraded for inconsistency.
‡‡Overall information size of 1213 was not achieved in either group and the overall effect estimate included one, hence downgraded for imprecision.
RCT, randomised controlled trials; RR, risk ratio.
Discussion
With the existing recommendation of USFDA for compassionate use of remdesivir in patients with COVID-19, it is being used worldwide. Current systematic review was planned for formulating recommendation from RCTs evaluating the efficacy of remdesivir in patients with COVID-19.
In the current systematic review, the OR for mortality failed to show any significant mortality benefit with the use of remdesivir. WHO solidarity trial14 showed no mortality benefit with the use of remdesivir. Though it was an open-label study, it is less likely to have bias in assessment of objective outcome like mortality. A total of 3451 patients were included in remdesivir and standard of care groups. Subgroup analysis revealed no mortality benefit in low risk (figure 3A—no oxygen requirement, online supplemental figure 2, no invasive ventilation) or high risk (figure 3A—oxygen requirement or assisted ventilation, online supplemental figure 2—invasive ventilation) group of patients with remdesivir.
At the time of recruitment, more patients were on invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO) in the placebo group. There were significantly more AEs reported in the control group in our review. This was due to the high incidence of serious AE in Beigel et al study.13 This is rare occurrence that serious AE were significantly more in control group. The fact that more severe patients were randomised into control group in the study by Beigel et al13 is the major reason for this finding. Similarly, the serious AE which also included the clinical events like renal failure and respiratory failure (5.2% in remdesivir and 8% in placebo arm) were also observed more in placebo group. Despite this imbalance, remdesivir was unable to show superiority in mortality rate.
Subgroup analysis of Beigel et al13 study revealed that remdesivir resulted in significant rate of clinical improvement in COVID-19 patients on oxygen therapy, while the patients not on oxygen, or on high flow oxygen or non-invasive ventilation and receiving mechanical ventilation or ECMO had similar clinical improvement as standard of care.
Placebo used in Beigel et al13 study was sulfo-butyl-ether b-cyclodextrin-sodium, used to dissolve remdesivir. The maximum recommended daily dose is approximately 250 mg/kg solvent used to dissolve remdesivir.13 The amount of solvent present in placebo was not quantified in protocol. Dose of solvent should be modified in patients with eGFR fall of more than 50% from baseline and is contraindicated in patients with eGFR less than 30 mL/min. But such modification were not done in either arms. Hence, the effect of solvent on patients with impaired renal function can be detrimental and cannot be ruled out.
In the study by Beigel et al,13 the median time of administration of drug from randomization was 9 days. Median recovery time from randomization was 11 days. In addition, 302 patients in remdesivir group did not receive 10 days of treatment. Therefore, it is difficult to infer that remdesivir resulted in recovery of patients, as an average of 2 days of administration of remdesivir resulting in complete recovery of patients seems implausible.
Virological cure is also an important outcome which was neglected by the authors. Wang et al reported no difference (percentage difference=−7.5 (95% CI −19.2 to 4.2)) in undetectable viral RNA load in remdesivir (75.6%) and placebo groups (83.1%). Patients may become asymptomatic but not cured. It has been observed that asymptomatic patients with RT-PCR positive test can have thromboembolic and chest CT changes. Study done by Merkler et al observed that 8 patients (26%) out of total 31 patients who had an ischaemic stroke were COVID-19 positive on RT-PCR testing. They did not have any COVID-19 symptoms on presentation.18 Silent hypoxaemia is a disturbing feature in asymptomatic patients with COVID-19 and has been found to be associated with poor outcomes.19
The Phase 3 SIMPLE trial4 results published in New England Journal of Medicine does not include a standard of care group. Similar to Beigel et al virological cure was not reported.4 Clinical status at day 14 was similar in 5-day course of remdesivir as compared with 10-day course. However, in comparison to standard care, 5-day group (OR 1.65 (95% CI 1.09 to 2.48); p=0.017) showed significant improvement while 10 day group did not (OR 1.31 (95% CI 0.88 to 1.95); p=0.18). Death reported on day 11 was similar in all three groups.20
In another study published by Grein et al5 in which compassionate use of remdesivir was done, did not have a control arm. Hence, the conclusion that remdesivir is effective cannot be drawn as the possibility of observing similar findings in control arm cannot be ruled out.
Three systematic reviews and meta-analysis were published on remdesivir.21–23 However, none of the reviews have included WHO solidarity trials in review. Exclusion of such large study (n=3451) decreases the power of systematic reviews. Our results are different from all three systematic reviews as Wilt et al23 and Shrestha et al21 have concluded mortality benefit with remdesivir while Elsawah et al22 concluded significant clinical improvement with remdesivir as compared with standard care. Meta-analysis performed by Solidarity trial group14 has shown no mortality benefit (OR=0.91, 95% CI 0.79 to 1.05), similar to our review. They did not perform meta-analysis with regard to other clinical endpoints and safety outcomes. Also, ROB-2 analysis of included RCTs and GRADE analysis was not applied.
The cost of the drug is US$2340 per patient but with no mortality benefit.24 According to World Bank data, low and lower middle income countries have Gross National Income per capita less than or equal to $ 1035 and between US$1036 to US$4045, respectively.25 As per WHO Global Health Expenditure database,26 current health expenditure per capita in low and lower middle income countries is less than US$36 and US$86, respectively.26 Therefore, from a cost–benefit perspective, we are of the opinion that their use should not be recommended, especially in low and lower middle income countries. In case of limited use, strict evidence-based guidelines should be followed for optimum health benefits. The cost of the drug will put extra burden on government by increasing the health cost without any benefit. Injudicious use of remdesivir without mortality benefit may also lead to increased incidence of AEs.
Limitations and strengths
A major strength of our systematic review is that four RCTs were included in our analysis with total sample size of 7324 patients. Study done by Wang et al and WHO Solidarity trial has low ROB. Robust method of analysis using ROB-2 and GRADE analysis is another strength of the current systematic review.
Quality of evidence: (GRADE)
The overall quality of systematic review is “Moderate”. Critical outcomes like mortality has moderate quality evidence. Clinical improvement was regarded as “Low”. Time to clinical improvement has “Very low” quality of evidence. Time to clinical improvement was used by regulatory agencies like US FDA for giving approval to remdesivir for treatment of patients with severe COVID-19. However, the quality of evidence for time to clinical improvement cannot be overlooked. This evidence suggests that further research is very likely to have an important impact on our confidence in the estimate of time to clinical improvement and likely to change the estimate. Moderate quality of evidence with regard to mortality showed that further RCTs are likely to have important impact and may change the no mortality benefit conclusion drawn from review.
Conclusion
Evidence of our systematic review indicates no benefit in mortality rate with remdesivir, with moderate quality of evidence. Benefit does exist in terms of rates of clinical improvement and faster time to clinical improvement in favour of remdesivir, but the evidence is of low and very low quality, respectively. Significant decrease in serious AEs as compared with placebo strengthens the evidence of more serious patients in placebo arm. No difference was shown in respiratory failure in the two groups (very low quality evidence). All outcomes except mortality in our meta-analysis were influenced by Beigel et al and Spinner et al, which has high ROB. WHO solidarity trial and Wang et al showed no mortality benefit, both having overall low ROB.
Supplementary Material
Acknowledgments
We acknowledge GRADE Pro team [McMaster University and Evidence Prime Inc. available from: https://gradepro.org/] for letting us use the software for the synthesis and overall assessment and grading of systematic review.
Footnotes
SS, DK and AC contributed equally.
Contributors: Study design and planning of systematic review: all authors. Literature search: AC, SS, PSK. Figures: SS, VKC, AC. Tables: DK, SS. Data collection and analysis: SS, DK. ROB: DK, AC, SS, query resolved by all authors. GRADE analysis: SS, AC, DK, Query resolved by all authors. Data interpretation: SS, DK, AC. Writing: SS, DK, AC, PSK. Corrections and final approval of manuscript: all of the authors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The datasets used and/or analyzed during the current study are available from the corresponding author on request.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–2. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020;395:1569–78. 10.1016/S0140-6736(20)31022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med 2020;383:1827–37. 10.1056/NEJMoa2015301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grein J, Ohmagari N, Shin D, et al. Compassionate use of Remdesivir for patients with severe Covid-19. N Engl J Med 2020;382:2327–36. 10.1056/NEJMoa2007016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGuinness LA, Higgins JPT. Risk‐of‐bias visualization (robvis): an R package and shiny web APP for visualizing risk‐of‐bias assessments. Res Synth Methods 2021;12:55–61. 10.1002/jrsm.1411 [DOI] [PubMed] [Google Scholar]
- 8.The Cochrane Collaboration. . Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, 2020. [Google Scholar]
- 9.Higgins J, Green S. Cochrane Handbook for systematic reviews of interventions: The Cochrane Collaboration, 2011. Available: www.handbook.cochrane.org
- 10.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001;135:982–9. 10.7326/0003-4819-135-11-200112040-00010 [DOI] [PubMed] [Google Scholar]
- 12.Schünemann H, Brożek J, Guyatt G. Grade Handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The grade Working group, 2013. Available: guidelinedevelopment.org/handbook
- 13.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med 2020;383:1813–26. 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO Solidarity Trial Consortium, Pan H, Peto R, et al. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med 2021;384:497–511. 10.1056/NEJMoa2023184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of Remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA 2020;324:1048–57. 10.1001/jama.2020.16349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulangu S, Dodd LE, Davey RT, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med 2019;381:2293–303. 10.1056/NEJMoa1910993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olender SA, Perez KK, AS G. Remdesivir for severe COVID-19 versus a cohort receiving standard of care. Clinical infectious disease in press;2020. 10.1093/cid/ciaa1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merkler AE, Parikh NS, Mir S, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol 2020. 10.1001/jamaneurol.2020.2730. [Epub ahead of print: 02 Jul 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brouqui P, Amrane S, Million M, et al. Asymptomatic hypoxia in COVID-19 is associated with poor outcome. Int J Infect Dis 2021;102:233–8. 10.1016/j.ijid.2020.10.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilead Announces results from phase 3 trial of Remdesivir in patients with moderate COVID-19. Available: https://www.gilead.com/news-and-press/press-room/press-releases/2020/6/gilead-announces-results-from-phase-3-trial-of-remdesivir-in-patients-with-moderate-covid-19
- 21.Shrestha DB, Budhathoki P, Syed N-I-H, et al. Remdesivir: a potential game-changer or just a myth? A systematic review and meta-analysis. Life Sci 2021;264:118663. 10.1016/j.lfs.2020.118663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elsawah HK, Elsokary MA, Abdallah MS. Efficacy and safety of remdesivir in hospitalized Covid-19 patients: systematic review and meta-analysis including network meta-analysis. Rev Med Virol 2020;31:e2187. 10.1002/rmv.2187 [DOI] [PubMed] [Google Scholar]
- 23.Wilt TJ, Kaka AS, MacDonald R, et al. Remdesivir for Adults With COVID-19 : A Living Systematic Review for American College of Physicians Practice Points. Ann Intern Med 2021;174:209–20. 10.7326/M20-5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rees V. Gilead prices remdesivir at $2,340 per patient for developed countries. European Pharmaceutical Review. Available: https://www.europeanpharmaceuticalreview.com/news/122592/gilead-prices-remdesivir-at-2340-per-patient-for-developed-countries/ [Accessed 30 Jun 2020].
- 25.World Bank, World Development Indicators, World Bank Country and lending groups , 2021. Available: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups#:~:text=For%20the%20current%202021%20fiscal,those%20with%20a%20GNI%20per
- 26.World Bank, World Development Indicators . World Health Organization Global Health Expenditure database (apps.who.int/nha/database), 2018. Available: https://data.worldbank.org/indicator/SH.XPD.CHEX.PC.CD?locations=XM
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-048416supp003.pdf (101.8KB, pdf)
bmjopen-2020-048416supp001.pdf (854.5KB, pdf)
bmjopen-2020-048416supp002.pdf (779.8KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The datasets used and/or analyzed during the current study are available from the corresponding author on request.