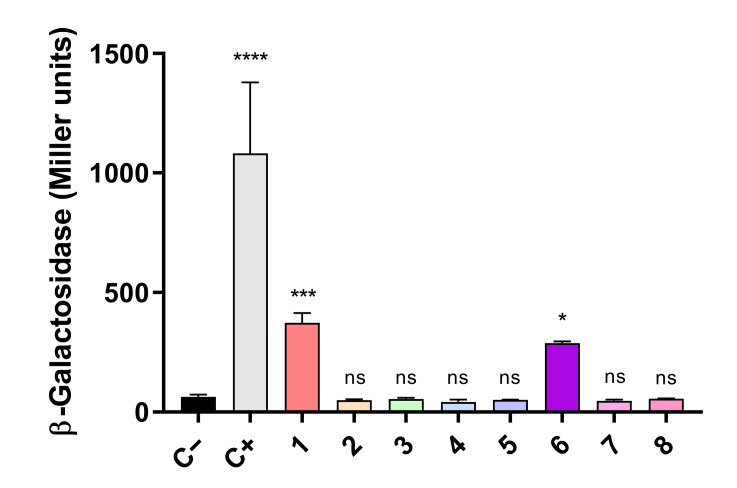
Figure 2.
Bacterial two-hybrid assays between FurA and Thioredoxin A. In vivo interaction between FurA and TrxA was quantified by measuring β-galactosidase activities in E. coli BTH101 cells harboring a pair of compatible plasmids with corresponding genes fused with the T25 or T18 adenylate cyclase domains: 1: pKNT25-trxA + pUT18-furA; 2: pKNT25-trxA + pUT18C-furA; 3: pKT25-trxA + pUT18-furA; 4: pKT25-trxA + pUT18C-furA; 5 pUT18-trxA + pKNT25-furA; 6: pUT18-trxA + pKT25-furA; 7 pUT18C-trxA + pKNT25-furA; and 8: pUT18C-trxA + pKT25-furA. Empty pKT25 and pUT18C served as negative controls (C−), whereas pKT25-zip and pUT18C-zip served as positive controls (C+). The β-galactosidase activity represents the averages ± standard deviation of three biological replicates. All β-galactosidase activity values were subjected to a one-way analysis of variance (ANOVA) test with respect to the negative control (C−) to determine if values were significant (ns not significant, * p < 0.05, *** p < 0.001, **** p < 0.0001).