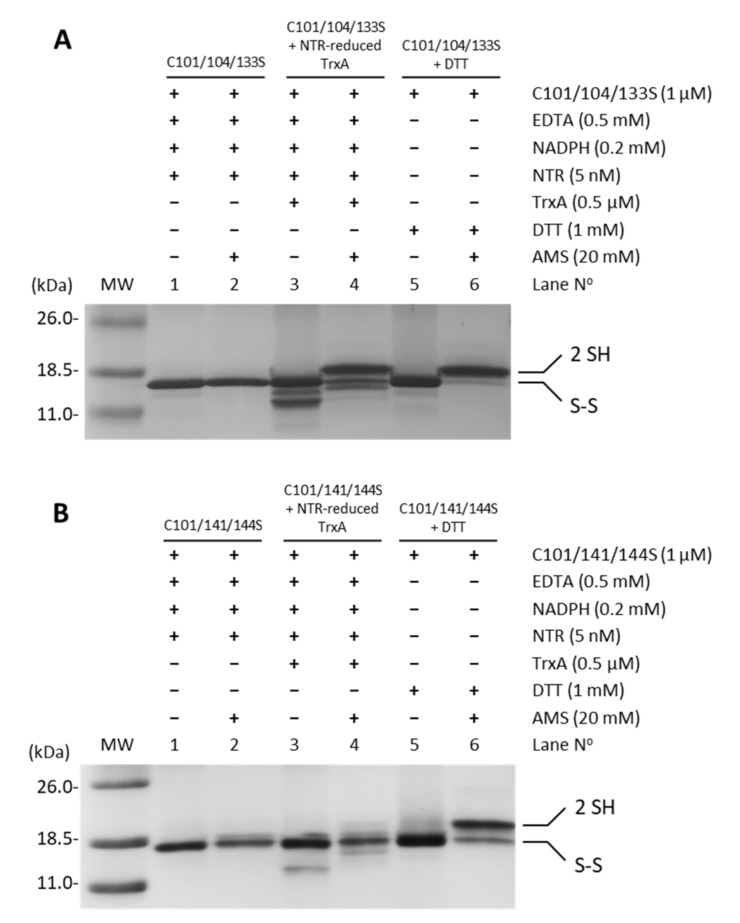
Figure 4.
Reduction of disulfide bridges C141-C144 and C104-C133 of FurA by TrxA. One micromolar of FurA triple cysteine mutants C101/104/133S (A) and C101/104/133S (B) were incubated with 0.5 µM TrxA in the presence of 0.2 mM NADPH, 0.5 mM EDTA, and 5 nM NTR to assess their reduction by NTR-reduced TrxA. FurA triple cysteine mutants were also incubated with 0.2 mM NADPH and 5 nM NRT to determine whether NADPH-reduced NTR affected their redox status and with 1 mM DTT as a positive control for protein reduction. Proteins were then precipitated with 10% (w/v) TCA, incubated with 20 mM AMS, loaded on non-reducing 15% SDS–polyacrylamide gels and stained with Coomassie.