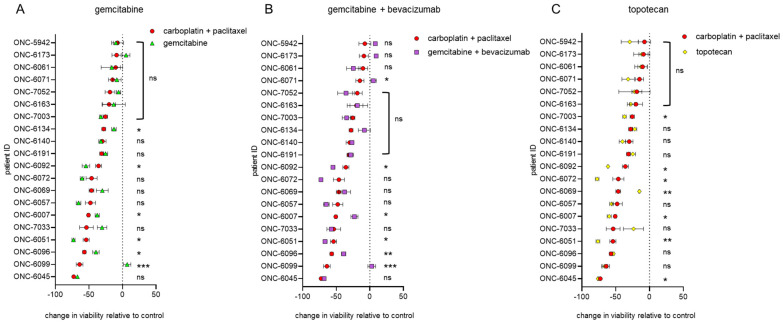
Figure 2.
Therapeutic efficacy of common second-line therapies for endometrial and ovarian cancers using PDO models. Endometrial and ovarian cancer PDO response to second-line therapies: (A) gemcitabine, (B) gemcitabine + bevacizumab and (C) topotecan. PDO models were treated with the indicated agents for 72 h, followed by assessment of cell viability as in Figure 1. The data were calculated as the change in viability relative to the control, which was set at 100% (i.e., no cell death). Each panel includes response to the standard chemotherapy (carboplatin + paclitaxel, red circles) for comparison, and the samples are ordered based on increasing sensitivity to carboplatin + paclitaxel. Statistical significance was assessed by two-way ANOVA with the Greenhouse–Geisser correction and Tukey’s multiple comparison test. Significant differences vs. carboplatin + paclitaxel are annotated on each panel; ns: not significant; * p < 0.05; ** p < 0.01; *** p < 0.001. All the statistical comparisons are provided in Supplementary Table S2, including specific p-values and comparisons vs. the untreated control. Note that some cases have greater sensitivity to second-line treatments as compared to the standard chemotherapy, whereas others are more resistant. For example, ONC-6099 is relatively sensitive to chemotherapy but had no change in viability when exposed to gemcitabine (panel A).