Abstract
Background
Escherichia coli is the most common cause of bloodstream infections (BSIs) and mortality is an important aspect of burden of disease. Using a multinational population-based cohort of E. coli BSIs, our objectives were to evaluate 30-day case fatality risk and mortality rate, and determine factors associated with each.
Methods
During 2014–2018, we identified 30-day deaths from all incident E. coli BSIs from surveillance nationally in Finland, and regionally in Sweden (Skaraborg) and Canada (Calgary, Sherbrooke, western interior). We used a multivariable logistic regression model to estimate factors associated with 30-day case fatality risk. The explanatory variables considered for inclusion were year (2014–2018), region (five areas), age (< 70-years-old, ≥70-years-old), sex (female, male), third-generation cephalosporin (3GC) resistance (susceptible, resistant), and location of onset (community-onset, hospital-onset). The European Union 28-country 2018 population was used to directly age and sex standardize mortality rates. We used a multivariable Poisson model to estimate factors associated with mortality rate, and year, region, age and sex were considered for inclusion.
Results
From 38.7 million person-years of surveillance, we identified 2961 30-day deaths in 30,923 incident E. coli BSIs. The overall 30-day case fatality risk was 9.6% (2961/30923). Calgary, Skaraborg, and western interior had significantly increased odds of 30-day mortality compared to Finland. Hospital-onset and 3GC-resistant E. coli BSIs had significantly increased odds of mortality compared to community-onset and 3GC-susceptible. The significant association between age and odds of mortality varied with sex, and contrasts were used to interpret this interaction relationship. The overall standardized 30-day mortality rate was 8.5 deaths/100,000 person-years. Sherbrooke had a significantly lower 30-day mortality rate compared to Finland. Patients that were either ≥70-years-old or male both experienced significantly higher mortality rates than those < 70-years-old or female.
Conclusions
In our study populations, region, age, and sex were significantly associated with both 30-day case fatality risk and mortality rate. Additionally, 3GC resistance and location of onset were significantly associated with 30-day case fatality risk. Escherichia coli BSIs caused a considerable burden of disease from 30-day mortality. When analyzing population-based mortality data, it is important to explore mortality through two lenses, mortality rate and case fatality risk.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-021-06326-x.
Keywords: Population-based, Bloodstream infection, Bacteremia, Escherichia coli, Mortality, Mortality rate, Case fatality
Background
Escherichia coli bloodstream infections (BSIs) are associated with an important burden of disease from mortality, because E. coli is the most common cause of bacterial BSIs [1–3]. In order to better understand the contribution of mortality to the overall burden of disease from E. coli BSIs, population-based studies are required [4]. Two general approaches for analyzing mortality data from population-based studies include evaluation of mortality rates and case fatality risks, which provide distinct yet complementary results [3, 5]. Mortality rates (number of deaths per population in a given time period) provide insight into the disease burden and case fatality risks (number of deaths per total number of cases) are markers of disease severity [5]. Appropriate comparison of mortality rates and case fatality risks from different bacterial causes of BSIs can help facilitate prioritization of funding, research, and surveillance.
A small number of published population-based studies have reported mortality data for E. coli BSIs, however, they did not all use the same definition of mortality [1, 6–11]. All-cause mortality definitions used included in-hospital mortality, in-hospital 7-day mortality, and 30-day mortality [1, 6–11]. The reported in-hospital case fatality risks for E. coli BSIs ranged from 8.9–11% [6, 7, 9]. One study reported an in-hospital 7-day case fatality risk of 5% for E. coli BSIs [8] and three studies reported 30-day case fatality risks between 8 and 18.2% [1, 10, 11]. Mortality rates for E. coli BSIs were only reported by three studies [3] (in-hospital mortality rate of 2.9 deaths/100,000 person-years, and 30-day mortality rates of 7 and 10.3 deaths/100,000 person-years) [6, 10, 11].
Antimicrobial-resistant E. coli BSIs have been associated with increased mortality and three population-based studies explored this association [6, 9, 11]. Ciprofloxacin-resistant [6, 11] and extended-spectrum β-lactamase (ESBL) producing [9] E. coli BSIs were associated with higher in-hospital mortality. Three previous population-based studies demonstrated significantly increased mortality in patients with hospital-onset E. coli BSIs (in-hospital mortality [6, 7] and 30-day mortality [11]). Multidrug resistant E. coli BSIs, which can include resistance to carbapenems and are associated with hospital-onset and healthcare-associated BSIs, can have significant impacts on the ability to successfully treat infections and prevent mortality [12–16]. Previous population-based studies reported mortality data for E. coli BSIs that were diagnosed prior to 2015, which highlights the opportunity to report more recent mortality data. We are not aware of a previously published multinational population-based study exploring mortality in E. coli BSIs.
Using data from a multinational population-based cohort of E. coli BSIs, we had two main objectives for our study: first, to evaluate the 30-day case fatality risk and factors associated with case fatality risk; and second, to evaluate the 30-day mortality rate and factors associated with mortality rate.
Methods
Surveillance populations and study protocol
We enrolled five areas from the International Bacteremia Surveillance Collaborative in three countries and two continents for this population-based cohort study [17]. The surveillance areas (2018 population) included: Calgary Health Region, Canada (1.7 million), country of Finland (5.5 million), Sherbrooke Region, Canada, (166,000), Skaraborg County Health Region, Sweden (267,000), and western interior area of British Columbia, Canada (191,000). Previous publications outline each area’s population and surveillance methodology [17–19]; Skaraborg now has two hospitals instead of four as previously published. Each area’s surveillance database captures at least 99% of all positive blood cultures from residents [17, 19]. The 5-year study (01/01/2014–12/31/2018) included all incident E. coli BSIs from area residents and we defined incident as the first E. coli isolate cultured from blood per patient per running year (at least 1 year of time elapsed between E. coli BSIs). Depending on the area, we retrieved data from electronic medical records or national registers (infectious disease, population, and hospital discharge registers), including: 30-day and 7-day all-cause mortality, year of culture, patient’s sex and age category (< 1 year, 1–9 years, then deciles until ≥90 years), location of onset (hospital-onset or community-onset), and susceptibility to third-generation cephalosporins (3GC). All-cause 30-day mortality was defined as death due to any reason within 30-days after the positive E. coli blood culture. We categorized BSIs as hospital-onset if the first positive blood culture was obtained at least 48 h after hospital admission or within 48 h of discharge, otherwise they were categorized as community-onset [20]. Each area used their own protocols for susceptibility testing. Using standardized templates, areas collected and compiled their own data. The study was approved by the Interior Health Research Ethics Board and University of Guelph Research Ethics Board, and individual informed consent waivers were granted (2013–14-052-I and 2018–10-050, respectively). As appropriate, local and / or national ethics requirements were adhered to by all other areas. All research methods were completed in accordance with the Declaration of Helsinki. Analyses related to incidence rates and antimicrobial resistance for E. coli BSIs in this study are documented in a separate manuscript [21].
Statistical analyses for case fatality risks
We performed statistical analyses in Stata 15.1 [22]. Based on E. coli BSI patients with 30-day mortality, we calculated proportions to summarize dichotomous variables (sex, location of onset, and 3GC resistance) and to summarize age, we determined the age category that contained the median 30-day death. Using univariable logistic regression, an odds ratio (OR) was estimated to compare the odds of a BSI being hospital-onset in 3GC-resistant (R) and 3GC-susceptible (S) E. coli BSI patients with 30-day mortality. We calculated 30-day case fatality risk by dividing the number of 30-day deaths by the number of incident E. coli BSIs. Thirty-day case fatality risks were calculated for each level of the dichotomous variables: age (< 70-years-old and ≥ 70-years-old), sex, location of onset, and 3GC resistance.
We used a logistic regression model to determine factors significantly associated with 30-day mortality in E. coli BSIs. The six categorical explanatory variables considered for inclusion in the logistic regression model included: year (2014 through 2018), region (five study areas), age (< 70-years-old and ≥ 70-years-old), sex (female and male), 3GC resistance (S and R), and location of onset (community-onset and hospital-onset). First, we completed univariable analysis and checked for correlation between explanatory variables using a Phi coefficient (ρ ≥ |0.8| was used as the threshold value) followed by building the multivariable logistic regression model starting with all of the explanatory variables. Three interaction effects were considered for inclusion in the final multivariable model: year and region, age and sex, and 3GC resistance and location of onset. In order to remain in the final multivariable model, variables had to meet at least one of the following criteria: be statistically significant (α = 0.05), be part of a significant interaction term, or be a confounding variable (based on > 20% change in another variable’s coefficient and meeting causal criteria) [23]. We assessed the final multivariable model for goodness-of-fit using a Hosmer-Lemeshow goodness-of-fit test [23]. We assessed Pearson standardized residuals, leverage, and influence statistics (delta-beta, delta-chi2, and delta-deviance) for each covariate pattern or observation [23]. If interaction terms were included in the final multivariable model, contrasts were used to interpret these relationships. Odds ratios (OR) were reported with 95% CI.
Statistical analyses for mortality rates
We calculated 30-day mortality rate by dividing the number of 30-day deaths by the population during the study period (from individual area census data). Direct age and sex standardization of the 30-day mortality rate to the European Union 28-country (EU28) 2018 population was performed to allow comparison of mortality rates between different regions and different years [22, 24]. The process above was repeated for 3GC-R and 3GC-S 30-day mortality data.
To identify factors significantly associated with E. coli BSI 30-day mortality rates, we used a Poisson regression model [22, 23]. The four categorical explanatory variables considered for inclusion in the Poisson regression model, included: year (2014 through 2018), region (five study areas), age (< 70-years-old and ≥ 70-years-old), and sex (female and male). We performed univariable analysis and tested for correlation between explanatory variables (as described for the logistic regression model) before placing all explanatory variables in the multivariable Poisson regression model. Interaction effects between year and region, and age and sex were considered for inclusion in the final multivariable model. In order to remain in the final multivariable model, variables had to meet at least one of the following criteria: be statistically significant (α = 0.05), be part of a significant interaction term, or be a confounding variable (based on > 20% change in another variable’s coefficient and meeting causal criteria) [23]. We assessed the fit of the final multivariable Poisson regression model by assessing the normality of Anscombe residuals and the significance of the overdispersion parameter when the model was re-fit as a negative binomial regression model (null-hypothesis that alpha = 0; p > 0.05 indicating the Poisson model fits the data) [23]. We assessed residuals (Pearson and deviance), leverage, and an influence statistic (Cook’s distance) for each covariate pattern or observation [23]. Incidence rate ratios (IRR) were reported with 95% confidence intervals (CI).
Results
During our 5-year study, there were 2961 30-day deaths in 30,923 incident E. coli BSIs, which we identified from 38.7 million person-years of surveillance. There was a left skewed age distribution, and the median age range of patients that experienced 30-day mortality was 80–89-years-old (Fig. 1). Thirty-day deaths in patients with E. coli BSIs were evenly distributed between the sexes (female 50.3%, 1490/2961). We found 11.4% (336/2961) of the E. coli BSIs that resulted in 30-day deaths were resistant to 3GC and this ranged from 3.5% (5/142) in Skaraborg to 24.2% (109/450) in Calgary. Most of the E. coli BSIs that resulted in 30-day deaths were community-onset E. coli BSIs (67.1%, 1987/2961); this proportion was lowest in Finland (65.4%, 1472/2252) and highest in Skaraborg (78.2%, 111/142). When considering only the E. coli BSI patients with 30-day mortality, those with 3GC-R E. coli BSIs were at greater odds of being hospital-onset compared to 3GC-S (OR:1.51, 95%CI:1.20–1.91, p < 0.001). Regional data for 3GC resistance and location of onset is available in Supplementary table 1, Additional file 1.
Fig. 1.
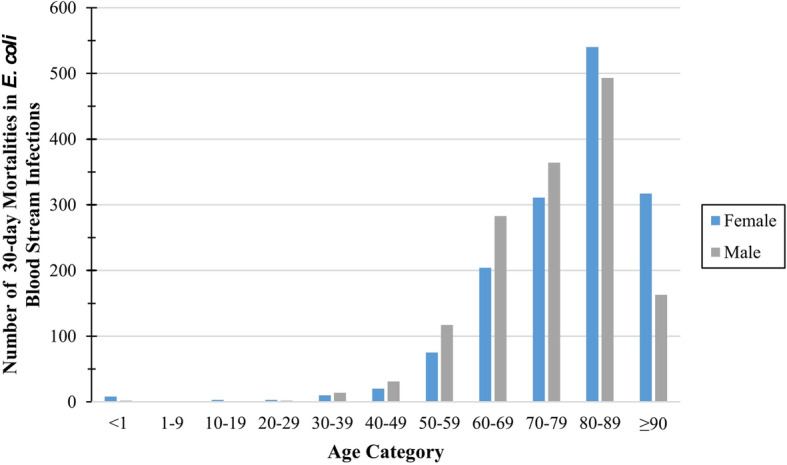
Number of 30-day deaths in E. coli bloodstream infection patients by age category and sex
The overall 30-day case fatality risk was 9.6% (2961/30923) and ranged from 7.2% (43/596) in Sherbrooke to 12.8% (74/578) in western interior (see Supplementary table 2, Additional file 1). Patients ≥70-years-old experienced an 11.4% (2188/19277) 30-day case fatality risk compared to 6.6% (773/11646) in those < 70-years-old. Thirty-day case fatality risk was higher in males (11.7%, 1470/12598) compared to females (8.1%, 1490/18325). Patients with 3GC-R E. coli BSIs had a 14.1% 30-day case fatality risk (336/2376), which was higher than those with 3GC-S E. coli BSIs (9.2%, 2625/28547). Thirty-day case fatality risk in hospital-onset E. coli BSIs was 17.7% (974/5506) compared to 7.8% (1987/25417) in community-onset E. coli BSIs. Of the 30-day deaths, 50.4% (1493/2961) occurred within 7-days of BSI diagnosis, which ranged from 47.2% (1063/2252) in Finland to 68.9% (51/74) in western interior.
With univariable logistic regression analysis, region, location of onset, 3GC resistance, sex, and age were all significantly associated with 30-day mortality in E. coli BSIs (see Supplementary table 3, Additional file 1). Our multivariable logistic regression model for 30-day mortality included region, location of onset, 3GC resistance and an interaction between age and sex (Table 1). Calgary, Skaraborg, and western interior had higher odds of 30-day mortality compared to Finland (Table 1). Compared to community-onset E. coli BSIs, patients with hospital-onset E. coli BSIs had higher odds of 30-day mortality (Table 1). Patients with E. coli BSIs resistant to 3GC had higher odds of dying within 30 days compared to those with 3GC-S E. coli BSIs (Table 1). Regarding the interaction between sex and age, when males were compared to either females of the same age category or younger females, males had higher odds of 30-day mortality (Table 2). When older females were compared to either younger females or males, the older females had higher odds of 30-day mortality (Table 2). When the two age categories of males were compared, older males had increased odds of 30-day mortality (Table 2).
Table 1.
Multivariable logistic regression model results estimating associations between explanatory variables and E. coli BSI 30-day mortalitya
| Variable | aOR | 95% CI | p-value |
|---|---|---|---|
| Region | < 0.001b | ||
| Finland | 1.00 | referent | |
| Calgary | 1.52 | 1.36–1.71 | < 0.001 |
| Sherbrooke | 0.87 | 0.64–1.20 | 0.403 |
| Skaraborg | 1.31 | 1.09–1.57 | 0.004 |
| Western interior | 1.69 | 1.31–2.18 | < 0.001 |
| Location of Onset | |||
| Community-onset | 1.00 | referent | |
| Hospital-onset | 2.44 | 2.25–2.66 | < 0.001 |
| 3GC-R | |||
| Susceptible | 1.00 | referent | |
| Resistant | 1.37 | 1.20–1.55 | < 0.001 |
| Sex | |||
| Female | 1.00 | referent | |
| Male | 1.77c | 1.52–2.05 | < 0.001 |
| Age Category | |||
| < 70-years-old | 1.00 | referent | |
| ≥ 70-years-old | 2.21c | 1.95–2.52 | < 0.001 |
| Interaction - Sex and Age | |||
| Male and ≥ 70 | 0.73c | 0.62–0.87 | < 0.001 |
Abbreviations: BSI Bloodstream infection, aOR Adjusted odds ratio, CI Confidence interval
aModel fits the data based on Hosmer-Lemeshow goodness-of-fit test (p = 0.653)
bOverall p-value for variable estimated using a likelihood ratio test
cExponentiated coefficients are not true aOR due to interaction term – see contrasts in Table 2
Table 2.
Results for contrasts examining interactions between sex and age based on multivariable logistic regression modela
| Contrast Statement | aOR | 95% CI | p-value |
|---|---|---|---|
| Males < 70 compared to females < 70 | 1.77 | 1.52–2.05 | < 0.001 |
| Males ≥70 compared to females ≥70 | 1.30 | 1.18–1.42 | < 0.001 |
| Males ≥70 compared to females < 70 | 2.87 | 2.52–3.28 | < 0.001 |
| Females ≥70 compared to females < 70 | 2.21 | 1.95–2.52 | < 0.001 |
| Males ≥70 compared to males < 70 | 1.62 | 1.44–1.83 | < 0.001 |
| Females ≥70 compared to males < 70 | 1.25 | 1.11–1.41 | < 0.001 |
Abbreviations: aOR Adjusted odds ratio, CI Confidence interval
aMultivariable logistic regression model estimating the associations between the explanatory variables (region, location of onset, third-generation cephalosporin resistance, sex, and age) and 30-day mortality in E. coli bloodstream infection (Table 1)
The overall crude 30-day mortality rate was 7.7 deaths/100,000 person-years. Supplementary table 2, Additional file 1 contains regional mortality data, and crude rates. The overall directly age and sex standardized mortality rate was 8.5 deaths/100,000 person-years, which was lowest in Sherbrooke, 5.4 deaths/100,000 person-years, and highest in Skaraborg, 9.6 deaths/100,000 person-years (Fig. 2). The directly standardized 3GC-R mortality rate was 0.9 deaths/100,000 person-years, which ranged from 0.3 to 2.0 deaths/100,000 person-years (Sherbrooke and Skaraborg, and Calgary, respectively) (Fig. 2). The directly standardized overall and 3GC-R mortality rates were relatively stable over the five-year study (Fig. 3). Regional and annual standardized overall, 3GC-R, and 3GC-S mortality rates are presented in Supplementary table 4, Additional file 1.
Fig. 2.
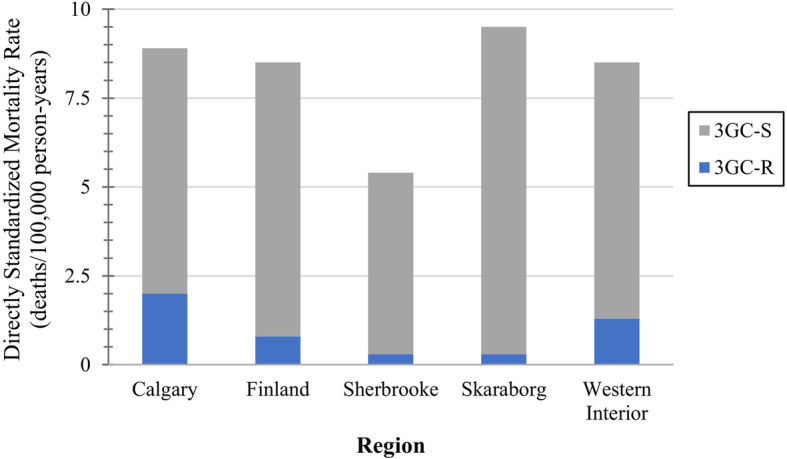
Directly age and sex standardized E. coli bloodstream infection 30-day mortality rates by areaa
Abbreviations: 3GC-R – Third-generation cephalosporin-resistant; 3GC-S – Third-generation cephalosporin-susceptible. aStandard population – EU28 2018 population
Fig. 3.
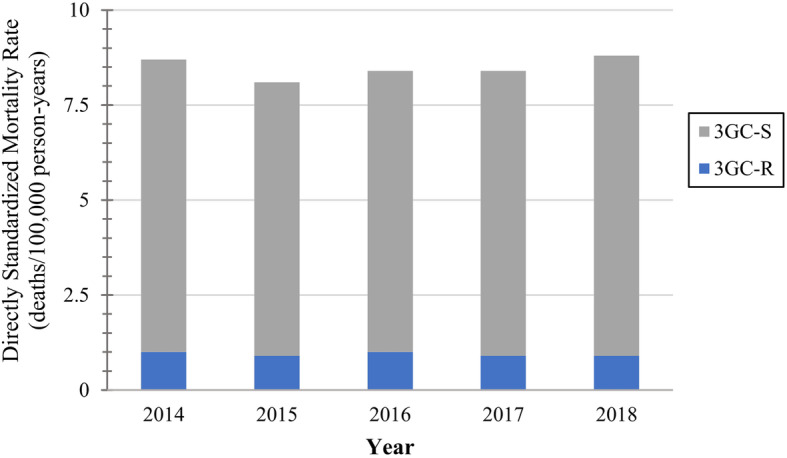
Directly age and sex standardized E. coli bloodstream infection 30-day mortality rates by yeara.
Abbreviations: 3GC-R – Third-generation cephalosporin-resistant; 3GC-S – Third-generation cephalosporin-susceptible. aStandard population – EU28 2018 population
With univariable Poisson regression analysis, region, and age were significantly associated with E. coli BSI 30-day mortality rate (see Supplementary table 5, Additional file 1). Our multivariable Poisson regression model for 30-day mortality rate included region, sex, and age (Table 3). Sherbrooke had a significantly lower 30-day mortality rate compared to Finland (Table 3). Compared to female E. coli BSI patients, male patients had a significantly higher 30-day mortality rate (Table 3). The 30-day mortality rate was significantly higher in patients that were ≥ 70-years-old compared to those < 70-years-old (Table 3).
Table 3.
Multivariable Poisson regression model results estimating associations between explanatory variables and E. coli BSI 30-day mortality ratea
| Variable | aIRR | 95% CI | p-value |
|---|---|---|---|
| Region | 0.035b | ||
| Finland | 1.00 | referent | |
| Calgary | 0.99 | 0.89–1.09 | 0.796 |
| Sherbrooke | 0.67 | 0.49–0.90 | 0.009 |
| Skaraborg | 1.14 | 0.96–1.35 | 0.138 |
| Western interior | 0.99 | 0.78–1.24 | 0.913 |
| Sex | |||
| Female | 1.00 | referent | |
| Male | 1.26 | 1.17–1.35 | < 0.001 |
| Age Category | |||
| < 70-years-old | 1.00 | referent | |
| ≥ 70-years-old | 20.35 | 18.73–22.11 | < 0.001 |
Abbreviations: BSI Bloodstream infection, aIRR Adjusted incidence rate ratio, CI Confidence interval
aModel fits the data based on normally distributed Anscombe residuals and lack of significant overdispersion (overdispersion parameter in negative binomial model [p = 0.302])
bOverall p-value for variable estimated using a likelihood ratio test
Discussion
Our population-based study provides important and novel insights into mortality in E. coli BSIs using data from five areas in three countries on two continents over a recent five-year period. Due to the population-based design of our cohort study, we were able to move beyond evaluation of case fatality risks to explore mortality rates. We used multivariable regression models to control for confounding variables while investigating risk factors for E. coli BSI mortality rate and case fatality risk.
Mortality is an important component of burden of disease in E. coli BSIs and was examined in only a limited number of previous population-based studies [1, 6–11]. We can cautiously compare our results to those from previously published population-based studies, but it is important to note that the studies are from different time periods and populations, and the studies used a variety of mortality definitions. In-hospital mortality has been shown to be a biased measure of mortality at a general population-level [25] and is likely influenced by factors associated with health system and healthcare delivery. Regional-population based studies from Canberra (Australia; 2000–2004; in-hospital 7-day mortality), Calgary (Canada; 2000–2006; in-hospital), mid-Norway (Norway; 2002–2013; 30-day), Auckland (New Zealand; 2005–2011; in-hospital), and two Thai provinces (Thailand; 2008–2014; in-hospital) reported case fatality risks of 5, 11, 8.6, 9, and 8.9%, respectively [6–10]. National surveillance in Finland (2004–2007) and England (07/2011–06/2012) reported 30-day case fatality risks of 8 and 18.2%, respectively [1, 11]. Our overall 30-day case fatality risk of 9.6% is similar to most of the case fatality risks reported in previous population-based studies. Non-population-based E. coli BSI studies reported extremely variable 30-day case fatality risks ranging from 2.8 to 37.5% [12–14, 26–38]; our 30-day case fatality risk is in the lower end of the range. However, the patients in the non-population-based studies are typically from highly selected populations including tertiary care centres, which consequently, provide minimal insight into the severity of E. coli BSIs at a general population-level.
We identified a 30-day crude mortality rate of 7.7 deaths/100,000 person-years, which was comparable to the 30-day crude mortality rate of 7 deaths/100,000 person-years from the mid-Norway study [10]. However, our rate was higher than that reported in a study from Calgary (in-hospital crude mortality rate, 2.9 deaths/100,000 person-years), and calculated for a study from Finland (30-day crude mortality rate, 3.5 deaths/100,000 person-years) [1, 6]. Our crude rate was lower than the 30-day crude mortality rate from England (10.3 deaths/100,000 person-years) [11].
When we have both case fatality risks and mortality rates from population-based studies, we can compare the results for E. coli BSIs to other bacterial causes of BSIs to understand their relative burden of disease. The comparisons are meant to be illustrative in nature and should be viewed with caution since the studies are from different populations, cover different time periods, in some cases use different mortality definitions, and are from different bacterial pathogens, which could be associated with different BSI and patient characteristics (e.g., BSI source, comorbidities, course of treatment). Ideally, our comparisons would be drawn from multinational studies from similar areas using the 30-day mortality definition with rates directly age and sex standardized to the same standard population, which is an area for future research. The mortality rates for other causes of BSIs presented below were calculated by multiplying the reported case fatality risk by the incidence rate [5] if they were not reported in the manuscript. If the definition of mortality was in-hospital mortality, then the mortality rate is an underestimate of the true population mortality rate [25]. For Staphylococcus aureus BSIs, studies from Finland (2004–2007, 30-day), Calgary (Canada, 2000–2006, in-hospital), and Skaraborg (Sweden, 2003–2005, 30-day) identified case fatality risks of 16.9, 25 and 19.1%, respectively, and mortality rates of 3.5, 4.9 and 5.9 deaths/100,000 person-years, respectively [1, 39, 40]. For Klebsiella spp. BSIs, a study from Olmsted county (Minnesota, USA, 1998–2007) reported a 28-day case fatality risk of 14% with associated mortality rate of 1.6 deaths/100,000 person-years [41], and a study from western interior (BC, Canada, 04/01/2010–03/31/2017) identified a 30-day case fatality risk of 18% and a mortality rate of 2.2 deaths/100,000 person-years [42]. A study from Calgary (Alberta, Canada, 2000–2006) evaluating Pseudomonas aeruginosa BSIs reported an in-hospital case fatality risk of 29% with a mortality rate of 1.0 deaths/100,000 person-years [43]. For β-hemolytic streptococcal BSIs, a study from western interior (BC, Canada, 2011–2018) identified a 30-day case fatality risk of 11% and a mortality rate of 1.6 deaths/100,000 person-years [44]. If we view case fatality risk in isolation, our 30-day case fatality risk of 9.6% for E. coli BSIs is lower than the case fatality risks for other causes. Therefore, when using case fatality risk as the sole measure of disease severity [5], we might conclude that E. coli BSIs are less severe compared to the other bacterial causes of BSIs. However, if we compare our crude E. coli BSI mortality rate of 7.7 deaths/100,000 person-years to the other studies, we note that our mortality rate is the highest. Escherichia coli BSIs pose a significant burden of disease through mortality because they are much more common, which results in high incidence and mortality rates. This highlights the importance of exploring mortality through two lenses, rate and case fatality risk, which is only possible at a general population level when we use population-based study designs.
By presenting mortality through rates and case fatality risks, we had the ability to use different regression modelling approaches. Logistic regression is an approach for modelling mortality that is commonly reported in the literature [23, 27, 31, 33, 35, 36, 45–48] and by using this method we evaluated factors associated with the proportion of deaths or case fatality risk. Additionally, by using the Poisson regression model [23], we were able to explore factors associated with the number of 30-day deaths in E. coli BSIs adjusted for the underlying population-at-risk, which is the mortality rate. Therefore, we were able to gain insight into the factors impacting two measures of mortality and we found that the drivers for mortality rate and case fatality risk were similar. The explanatory variables that were associated with both the 30-day mortality rate and 30-day case fatality risk were region, age category, and sex although there was an interaction between age and sex in the model for 30-day case fatality risk. Additionally, location of onset, and 3GC resistance were associated with 30-day case fatality risk. The number of explanatory variables for the Poisson regression model were limited because population data were only available stratified by year, age and sex. Even in light of the data limitations affecting mortality rate modelling, it still provides important insight into burden of disease and can easily be added to the analyses of future population-based studies.
Hospital-onset and 3GC-R E. coli BSIs both significantly increased the odds of 30-day mortality. These findings are consistent with previous studies (Hospital-onset [6, 7, 14, 33], and 3GC-R [9, 31, 33, 45]). A meta-analysis from a 2014 systematic review also identified a significant increase in the risk of dying within 30-days of BSI diagnosis, when patients had 3GC-R E. coli BSIs compared to those with 3GC-S E. coli BSIs [49]. Interventions could be directed at decreasing 3GC-R or hospital-onset E. coli BSIs in order to reduce 30-day mortality in patients with E. coli BSIs.
The demographic factors, age and sex, are known to be important factors related to mortality. We found that E. coli BSI patients ≥70-years-old and those that were male experienced 30-day mortality at a higher rate, which is consistent with previous general BSI studies that found the rate of mortality in BSIs was significantly higher with increasing age and in male patients [1, 10]. The relationship between age category and 30-day case fatality depended on the sex being considered. We did not identify a previous study that reported an interaction between age and sex, however there are reports of higher case fatality risks with increasing age [6, 11, 33, 36] and in males [1, 11, 14, 33, 35]. The significant interaction between age and sex reinforces the importance of considering interaction effects during multivariable model building.
There were significant regional differences in 30-day mortality rate and 30-day case fatality risk. However, since region is a proxy for other unmeasured variables, we are unable to propose explanations for these regional differences. However, by including region as a fixed variable in the multivariable regression models, we were able to control for the regional differences while estimating the association between the other factors, and either 30-day mortality rate or case fatality. For future research projects, more detailed information regarding specific regional and population variables could be collected, which would facilitate exploration of the regional differences. During our 5-year study, we did not find significant changes over time in 30-day mortality rate or case fatality risk. When we compare results from a previous Finnish study (2004–2007) [1] to our results from Finland (2014–2018), we see 30-day case fatality risks in E. coli BSIs of 8 and 9.1%, and 30-day crude mortality rates of 3.5 deaths/100,000 person-years (calculated) and 8.2 deaths/100,000 person-years, respectively. There is a 13.8% increase in case fatality risk and a 133% increase in the mortality rate between the two studies. Considering the small increase in case fatality risk, the large increase in mortality rate can be mostly attributed to a substantial increase in the E. coli BSI incidence rate between the two studies. This comparison is only based on one area but we may have identified increases in the mortality rate over time, if our study covered a longer time period.
Our study did have some limitations that should be noted. We were only able to report community-onset and not further characterize these community-onset BSI episodes into healthcare-acquired and community-acquired. Detailed data were not available for co-morbidities, source of BSI, additional antimicrobial susceptibility results, treatment, length of hospital stay prior to positive culture, or other burden of disease outcomes (e.g., length of hospital stay, hospital costs, or measures of morbidity). We did not have information on culturing rates. The results from our study should only be generalized to other high-income countries [50]. One population-based study from Thailand [9], an upper-middle-income country [50], presented E. coli BSI mortality data, but more population-based research in low, lower-middle, and upper-middle-income countries is required to understand from a global perspective, the contribution of mortality to the burden of disease from E. coli BSIs.
Conclusions
Our multinational population-based study identified that E. coli BSIs have a considerable burden of disease from 30-day mortality. Region, age, and sex were associated with both 30-day mortality rate and 30-day case fatality risk; in addition, 3GC resistance and location of onset were associated with 30-day case fatality risk. Because E. coli BSIs are much more common than other bacterial causes of BSIs, our population-based 30-day mortality rate was higher than that reported for other bacterial causes of BSIs, including Staphylococcus aureus, Klebsiella spp., β-hemolytic streptococci, and Pseudomonas aeruginosa. Our study highlights the importance of using population-based mortality data to evaluate mortality rate in addition to case fatality risk because they provide insights into different aspects of mortality. Even though E. coli BSIs have lower case fatality than some other bacterial causes of BSI and therefore are sometimes viewed as less severe, since E. coli is the most common cause of BSIs, it has a very substantial impact on human health as a result of mortality.
Supplementary Information
Additional file 1: Table S1. Proportion of E. coli bloodstream infection patients with 30-day mortality by region that were resistant to third-generation cephalosporins and location of onset. Table S2. Counts of 30-day mortality and incident E. coli bloodstream infections, length of patient follow-up, case fatality risks, and crude mortality rates. Table S3. Crude odds ratios for the univariable logistic regression models estimating associations between 30-day mortality in E. coli bloodstream infections, and region, year, location of onset, third-generation cephalosporin resistance, sex and age. Table S4. Directly age and sex standardized E. coli bloodstream infection mortality rates for overall, third-generation cephalosporin-resistant and susceptible E. coli bloodstream infections. Table S5. Crude incidence rate ratios for the univariable Poisson regression models estimating associations between E. coli bloodstream infection 30-day mortality rates, and region, year, sex and age.
Acknowledgements
GJ would like to thank Niklas Klaar and Ulrika Eriksson for help with data collection. LV would like to thank Anaïs Marcil-Héguy and Cynthia Grenier for help with data collection.
We thank members of the International Bacteremia Surveillance Collaborative for support of this project: Kevin B. Laupland (Kamloops, Canada and Brisbane, Australia); Daniel B. Gregson, Deirdre L. Church (Calgary, Canada); Louis Valiquette (Sherbrooke, Canada); Outi Lyytikainen (Helsinki, Finland); Peter Collignon, Karina J. Kennedy (Canberra, Australia); Gunnar Jacobsson (Skaraborg, Sweden); John Galbraith, Pamela Kibsey, Kennard Tan (Victoria, Canada); Henrik C. Schonheyder (Aalborg, Denmark); Jenny Dahl Knudsen, Jens Otto Jarlov, Christian Ostergaard Anderson, Mette Pinholt (Copenhagen, Denmark); Kim Oren Gradel (Odense, Denmark); and Frank Brunkhorst (Jena, Germany).
Abbreviations
- 3GC
Third-generation cephalosporin
- 3GC-R
Third-generation cephalosporin-resistant
- 3GC-S
Third-generation cephalosporin-susceptible
- BSI
Bloodstream infection
- BSIs
Bloodstream infections
- CI
Confidence interval
- E. coli
Escherichia coli
- ESBL
Extended-spectrum β-lactamase
- EU28
European Union 28-country
- IRR
Incidence rate ratio
- OR
Odds ratio
Authors’ contributions
MCM: contributed to data collection; conducted the data analyses; and drafted and edited the manuscript; KBL, OL, GJ, PC, DBG, and LV: contributed to data collection; and manuscript preparation; SAM and DLP: contributed to data analyses; and manuscript preparation. All authors read and approved the final version of the manuscript.
Funding
No external funding was received as direct support for this project. MCM’s PhD program was supported by a Banting and Charles Best Canadian Graduate Scholarship Doctoral Award from the Canadian Institutes of Health Research, a Brock Doctoral Scholarship from the University of Guelph, an OVC Scholarship from the Ontario Veterinary College at the University of Guelph and funding from the Genome Research Development Initiative through the Government of Canada. The funding organizations were not involved in any stage of the project.
Availability of data and materials
The aggregated datasets analyzed during the current study may be available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Interior Health and University of Guelph Research Ethics Boards approved the study and granted waivers of individual informed consent (2013–14-052-I and 2018–10-050, respectively). All other participating surveillance areas were approved by their local Research Ethics Boards.
Consent for publication
Not applicable.
Competing interests
MCM reports a scholarship from the Canadian Institutes of Health Research and funding for a Federal Student Work Experience Program placement from the Government of Canada – Genome Research Development Initiative, both related to her PhD program and outside the submitted work. LV reports being a stock holder of Lumed Inc., outside the submitted work. All other authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Skogberg K, Lyytikainen O, Ollgren J, Nuorti JP, Ruutu P. Population-based burden of bloodstream infections in Finland. Clin Microbiol Infect. 2012;18(6):E170–E176. doi: 10.1111/j.1469-0691.2012.03845.x. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen SL, Pedersen C, Jensen TG, Gradel KO, Kolmos HJ, Lassen AT. Decreasing incidence rates of bacteremia: a 9-year population-based study. J Inf Secur. 2014;69(1):51–59. doi: 10.1016/j.jinf.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Bonten M, Johnson JR, van den Biggelaar AHJ, Georgalis L, Geurtsen J, de Palacios PI, et al. Epidemiology of Escherichia coli bacteremia: a systematic literature review. Clin Infect Dis. 2020;72:1211. doi: 10.1093/cid/ciaa210. [DOI] [PubMed] [Google Scholar]
- 4.Laupland KB. Incidence of bloodstream infection: a review of population-based studies. Clin Microbiol Infect. 2013;19(6):492–500. doi: 10.1111/1469-0691.12144. [DOI] [PubMed] [Google Scholar]
- 5.Tom S, Galbraith JC, Valiquette L, Jacobsson G, Collignon P, Schonheyder HC, et al. Case fatality ratio and mortality rate trends of community-onset Staphylococcus aureus bacteraemia. Clin Microbiol Infect. 2014;20(10):O630–O632. doi: 10.1111/1469-0691.12564. [DOI] [PubMed] [Google Scholar]
- 6.Laupland KB, Gregson DB, Church DL, Ross T, Pitout JDD. Incidence, risk factors and outcomes of Escherichia coli bloodstream infections in a large Canadian region. Clin Microbiol Infect. 2008;14(11):1041–1047. doi: 10.1111/j.1469-0691.2008.02089.x. [DOI] [PubMed] [Google Scholar]
- 7.Williamson DA, Lim A, Wiles S, Roberts SA, Freeman JT. Population-based incidence and comparative demographics of community-associated and healthcare-associated Escherichia coli bloodstream infection in Auckland, New Zealand, 2005-2011. BMC Infect Dis. 2013;13(1):385. doi: 10.1186/1471-2334-13-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy KJ, Roberts JL, Collignon PJ. Escherichia coli bacteraemia in Canberra: incidence and clinical features. Med J Aust. 2008;188(4):209–213. doi: 10.5694/j.1326-5377.2008.tb01586.x. [DOI] [PubMed] [Google Scholar]
- 9.Sawatwong P, Sapchookul P, Whistler T, Gregory CJ, Sangwichian O, Makprasert S, Jorakate P, Srisaengchai P, Thamthitiwat S, Promkong C, Nanvatthanachod P, Vanaporn M, Rhodes J. High burden of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae bacteremia in older adults: a seven-year study in two rural Thai provinces. Am J Trop Med Hyg. 2019;100(4):943–951. doi: 10.4269/ajtmh.18-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehl A, Åsvold BO, Lydersen S, Paulsen J, Solligård E, Damås JK, Harthug S, Edna TH. Burden of bloodstream infection in an area of mid-Norway 2002-2013: a prospective population-based observational study. BMC Infect Dis. 2017;17(1):205. doi: 10.1186/s12879-017-2291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abernethy JK, Johnson AP, Guy R, Hinton N, Sheridan EA, Hope RJ. Thirty day all-cause mortality in patients with Escherichia coli bacteraemia in England. Clin Microbiol Infect. 2015;21(3):251.e1–251.e8. doi: 10.1016/j.cmi.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Cheong HS, Kang CI, Kwon KT, Heo ST, Wi YM, Kim ES, Lee JS, Ko KS, Chung DR, Lee NY, Song JH, Peck KR. Clinical significance of healthcare-associated infections in community-onset Escherichia coli bacteraemia. J Antimicrob Chemother. 2007;60(6):1355–1360. doi: 10.1093/jac/dkm378. [DOI] [PubMed] [Google Scholar]
- 13.Lim C, Takahashi E, Hongsuwan M, Wuthiekanun V, Thamlikitkul V, Hinjoy S, et al. Epidemiology and burden of multidrug-resistant bacterial infection in a developing country. elife. 2016;5(09):e18082. doi: 10.7554/eLife.18082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon EJ, Choi MH, Park YS, Lee HS, Kim D, Lee H, Shin KS, Shin JH, Uh Y, Kim YA, Shin JH, Jeong SH. Impact of host-pathogen-treatment tripartite components on early mortality of patients with Escherichia coli bloodstream infection: prospective observational study. EBioMedicine. 2018;35:76–86. doi: 10.1016/j.ebiom.2018.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komatsu Y, Kasahara K, Inoue T, Lee ST, Muratani T, Yano H, Kirita T, Mikasa K. Molecular epidemiology and clinical features of extended-spectrum beta-lactamase- or carbapenemase-producing Escherichia coli bacteremia in Japan. PLoS One. 2018;13(8):e0202276. doi: 10.1371/journal.pone.0202276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang HJ, Hsu PC, Yang CC, Kuo AJ, Chia JH, Wu TL, Lee MH. Risk factors and outcomes of carbapenem-nonsusceptible Escherichia coli bacteremia: a matched case-control study. J Microbiol Immunol Infect. 2011;44(2):125–130. doi: 10.1016/j.jmii.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Laupland KB, Schonheyder HC, Kennedy KJ, Lyytikainen O, Valiquette L, Galbraith J, et al. Rationale for and protocol of a multi-national population-based bacteremia surveillance collaborative. BMC Res Notes. 2009;2(1):146. doi: 10.1186/1756-0500-2-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laupland KB, Lyytikainen O, Sogaard M, Kennedy KJ, Knudsen JD, Ostergaard C, et al. The changing epidemiology of Staphylococcus aureus bloodstream infection: a multinational population-based surveillance study. Clin Microbiol Infect. 2013;19(5):465–471. doi: 10.1111/j.1469-0691.2012.03903.x. [DOI] [PubMed] [Google Scholar]
- 19.Laupland KB, Pasquill K, Parfitt EC, Naidu P, Steele L. Burden of community-onset bloodstream infections, Western interior, British Columbia. Canada Epidemiol Infect. 2016;144(11):2440–2446. doi: 10.1017/S0950268816000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laupland KB, Church DL. Population-based epidemiology and microbiology of community-onset bloodstream infections. Clin Microbiol Rev. 2014;27(4):647–664. doi: 10.1128/CMR.00002-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacKinnon MC. Exploring the burden of disease associated with human Escherichia coli bloodstream infections: University of Guelph. 2020. [Google Scholar]
- 22.StataCorp . Stata Statistical Software: Release 15. College Station: StataCorp LLC; 2017. [Google Scholar]
- 23.Dohoo IR, Martin SW, Stryhn H. Methods in epidemiologic research. Charlottetown: VER, Inc; 2012. [Google Scholar]
- 24.Eurostat. EU 28-country 2018 population on 1 January by age and sex 2019. Available from: https://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=demo_pjan&lang=en. Accessed 20 Nov 2019.
- 25.Laupland KB, Pasquill K, Parfitt EC, Dagasso G, Gupta K, Steele L. Inhospital death is a biased measure of fatal outcome from bloodstream infection. Clin Epidemiol. 2019;11:47–52. doi: 10.2147/CLEP.S187381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Kraker ME, Wolkewitz M, Davey PG, Koller W, Berger J, Nagler J, et al. Burden of antimicrobial resistance in European hospitals: excess mortality and length of hospital stay associated with bloodstream infections due to Escherichia coli resistant to third-generation cephalosporins. J Antimicrob Chemother. 2011;66(2):398–407. doi: 10.1093/jac/dkq412. [DOI] [PubMed] [Google Scholar]
- 27.Denis B, Lafaurie M, Donay JL, Fontaine JP, Oksenhendler E, Raffoux E, Hennequin C, Allez M, Socie G, Maziers N, Porcher R, Molina JM. Prevalence, risk factors, and impact on clinical outcome of extended-spectrum beta-lactamase-producing Escherichia coli bacteraemia: a five-year study. Int J Infect Dis. 2015;39:1–6. doi: 10.1016/j.ijid.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Freeman JT, McBride SJ, Nisbet MS, Gamble GD, Williamson DA, Taylor SL, et al. Bloodstream infection with extended-spectrum beta-lactamase-producing Enterobacteriaceae at a tertiary care hospital in New Zealand: risk factors and outcomes. Int J Infect Dis. 2012;16(5):e371–e374. doi: 10.1016/j.ijid.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Haruki Y, Hagiya H, Haruki M, Sugiyama T. Clinical characteristics and outcome of critically ill patients with bacteremia caused by extended-spectrum beta-lactamase-producing and non-producing Escherichia coli. J Infect Chemother. 2018;24(11):944–947. doi: 10.1016/j.jiac.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh CJ, Shen YH, Hwang KP. Clinical implications, risk factors and mortality following community-onset bacteremia caused by extended-spectrum beta-lactamase (ESBL) and non-ESBL producing Escherichia coli. J Microbiol Immunol Infect. 2010;43(3):240–248. doi: 10.1016/S1684-1182(10)60038-2. [DOI] [PubMed] [Google Scholar]
- 31.Kang CI, Song JH, Chung DR, Peck KR, Ko KS, Yeom JS, Ki HK, Son JS, Lee SS, Kim YS, Jung SI, Kim SW, Chang HH, Ryu SY, Kwon KT, Lee H, Moon C, Shin SY, Korean Network for Study of Infectious Diseases (KONSID) Risk factors and treatment outcomes of community-onset bacteraemia caused by extended-spectrum beta-lactamase-producing Escherichia coli. Int J Antimicrob Agents. 2010;36(3):284–287. doi: 10.1016/j.ijantimicag.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Martelius T, Jalava J, Karki T, Mottonen T, Ollgren J, Lyytikainen O, et al. Nosocomial bloodstream infections caused by Escherichia coli and Klebsiella pneumoniae resistant to third-generation cephalosporins, Finland, 1999-2013: trends, patient characteristics and mortality. Infect Dis (Lond) 2016;48(3):229–234. doi: 10.3109/23744235.2015.1109135. [DOI] [PubMed] [Google Scholar]
- 33.Melzer M, Petersen I. Mortality following bacteraemic infection caused by extended spectrum beta-lactamase (ESBL) producing E. coli compared to non-ESBL producing E. coli. J Inf Secur. 2007;55(3):254–259. doi: 10.1016/j.jinf.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Ortega M, Marco F, Soriano A, Almela M, Martinez JA, Munoz A, et al. Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic-resistant strain and their impact on the outcome. J Antimicrob Chemother. 2009;63(3):568–574. doi: 10.1093/jac/dkn514. [DOI] [PubMed] [Google Scholar]
- 35.Camins BC, Marschall J, DeVader SR, Maker DE, Hoffman MW, Fraser VJ. The clinical impact of fluoroquinolone resistance in patients with E. coli bacteremia. J Hosp Med. 2011;6(6):344–349. doi: 10.1002/jhm.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blandy O, Honeyford K, Gharbi M, Thomas A, Ramzan F, Ellington MJ, Hope R, Holmes AH, Johnson AP, Aylin P, Woodford N, Sriskandan S. Factors that impact on the burden of Escherichia coli bacteraemia: multivariable regression analysis of 2011-2015 data from West London. J Hosp Infect. 2019;101(2):120–128. doi: 10.1016/j.jhin.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 37.Mora-Rillo M, Fernández-Romero N, Navarro-San Francisco C, Díez-Sebastián J, Romero-Gómez MP, Fernández FA, et al. Impact of virulence genes on sepsis severity and survival in Escherichia coli bacteremia. Virulence. 2015;6(1):93–100. doi: 10.4161/21505594.2014.991234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giannella M, Pascale R, Toschi A, Ferraro G, Graziano E, Furii F, Bartoletti M, Tedeschi S, Ambretti S, Lewis RE, Viale P. Treatment duration for Escherichia coli bloodstream infection and outcomes: retrospective single-Centre study. Clin Microbiol Infect. 2018;24(10):1077–1083. doi: 10.1016/j.cmi.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Laupland KB, Ross T, Gregson DB. Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000-2006. J Infect Dis. 2008;198(3):336–343. doi: 10.1086/589717. [DOI] [PubMed] [Google Scholar]
- 40.Jacobsson G, Gustafsson E, Andersson R. Outcome for invasive Staphylococcus aureus infections. Eur J Clin Microbiol Infect Dis. 2008;27(9):839–848. doi: 10.1007/s10096-008-0515-5. [DOI] [PubMed] [Google Scholar]
- 41.Al-Hasan MN, Lahr BD, Eckel-Passow JE, Baddour LM. Epidemiology and outcome of Klebsiella species bloodstream infection: a population-based study. Mayo Clin Proc. 2010;85(2):139–144. doi: 10.4065/mcp.2009.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reid CB, Steele L, Pasquill K, Parfitt EC, Laupland KB. Occurrence and determinants of Klebsiella species bloodstream infection in the western interior of British Columbia. Canada BMC Infect Dis. 2019;19(1):1070. doi: 10.1186/s12879-019-4706-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parkins MD, Gregson DB, Pitout JD, Ross T, Laupland KB. Population-based study of the epidemiology and the risk factors for Pseudomonas aeruginosa bloodstream infection. Infection. 2010;38(1):25–32. doi: 10.1007/s15010-009-9145-9. [DOI] [PubMed] [Google Scholar]
- 44.Laupland KB, Pasquill K, Parfitt EC, Steele L. Bloodstream infection due to β-hemolytic streptococci: a population-based comparative analysis. Infection. 2019;47(6):1021–1025. doi: 10.1007/s15010-019-01356-9. [DOI] [PubMed] [Google Scholar]
- 45.Ha YE, Kang CI, Cha MK, Park SY, Wi YM, Chung DR, Peck KR, Lee NY, Song JH. Epidemiology and clinical outcomes of bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli in patients with cancer. Int J Antimicrob Agents. 2013;42(5):403–409. doi: 10.1016/j.ijantimicag.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 46.Park SH, Choi SM, Lee DG, Kim J, Choi JH, Kim SH, Kwon JC, Yoo JH. Emergence of extended-spectrum beta-lactamase-producing Escherichia coli as a cause of community-onset bacteremia in South Korea: risk factors and clinical outcomes. Microb Drug Resist. 2011;17(4):537–544. doi: 10.1089/mdr.2011.0072. [DOI] [PubMed] [Google Scholar]
- 47.Trecarichi EM, Tumbarello M, Spanu T, Caira M, Fianchi L, Chiusolo P, et al. Incidence and clinical impact of extended-spectrum-beta-lactamase (ESBL) production and fluoroquinolone resistance in bloodstream infections caused by Escherichia coli in patients with hematological malignancies. J Inf Secur. 2009;58(4):299–307. doi: 10.1016/j.jinf.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Parveen A, Sultan F, Raza A, Zafar W, Nizamuddin S, Mahboob A, Saleem S, Nazeer SH. Bacteraemia caused by Escherichia coli in cancer patients at a specialist center in Pakistan. J Pak Med Assoc. 2015;65(12):1271–1276. [PubMed] [Google Scholar]
- 49.World Health Organization Antimicrobial Resistance: Global Report on Surveillance. France. 2014;1:1–256. [Google Scholar]
- 50.The World Bank . World Bank Country and Lending Groups: Classification. 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Proportion of E. coli bloodstream infection patients with 30-day mortality by region that were resistant to third-generation cephalosporins and location of onset. Table S2. Counts of 30-day mortality and incident E. coli bloodstream infections, length of patient follow-up, case fatality risks, and crude mortality rates. Table S3. Crude odds ratios for the univariable logistic regression models estimating associations between 30-day mortality in E. coli bloodstream infections, and region, year, location of onset, third-generation cephalosporin resistance, sex and age. Table S4. Directly age and sex standardized E. coli bloodstream infection mortality rates for overall, third-generation cephalosporin-resistant and susceptible E. coli bloodstream infections. Table S5. Crude incidence rate ratios for the univariable Poisson regression models estimating associations between E. coli bloodstream infection 30-day mortality rates, and region, year, sex and age.
Data Availability Statement
The aggregated datasets analyzed during the current study may be available from the corresponding author on reasonable request.


