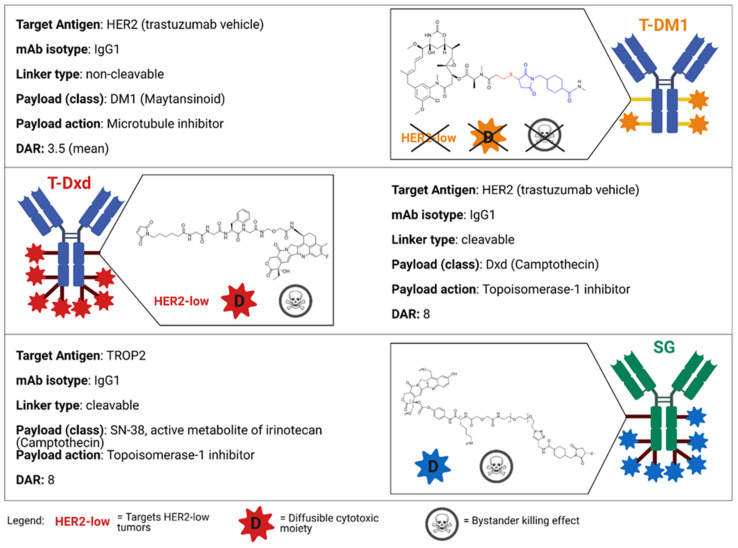
Figure 2.
Main features of ADCs currently FDA-approved for the treatment of BC. Abbreviations: T-DM1, trastuzumab emtansine; T-DXd, trastuzumab deruxtecan; SG, Sacituzumab govitecan; HER2, human epidermal growth factor receptor 2; mAb, monoclonal antibody; DAR, drug-to-antibody ratio; DM1, emtansine; DxD, deruxtecan; TROP2, Trophoblast cell surface antigen 2; TNBC, triple-negative breast cancer. Created with Biorender.com (accessed on 15 March 2021).